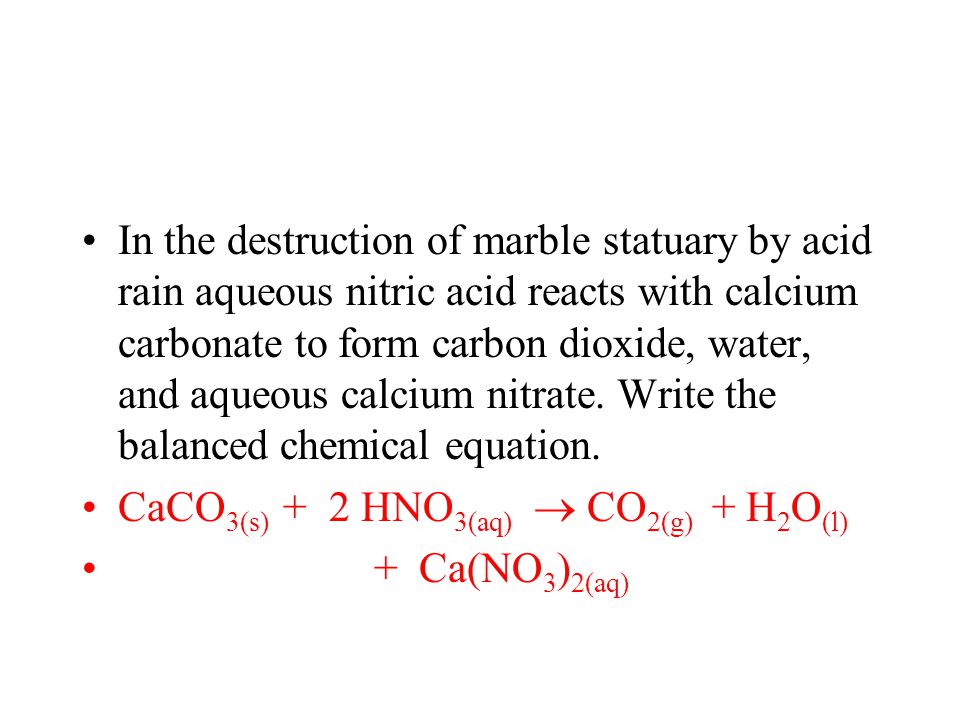Breathtaking Acid Rain Balanced Equation

Acidic rain is a complex mixture of nitrous nitric sulfurous and sulfuric acids which all combine to lower the pH.
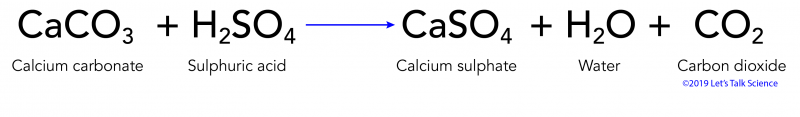
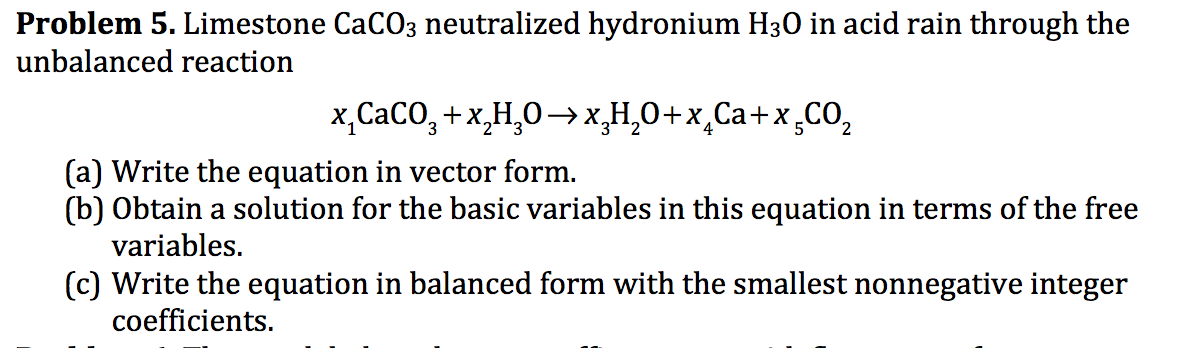
Acid rain balanced equation. Carbonic acid then dissociates to give the hydrogen ion H and the hydrogen carbonate ion HCO 3- Equation 2. Write a balanced chemical equation for this reaction. The reaction equation for acid rain produced from nitrogen oxides is.
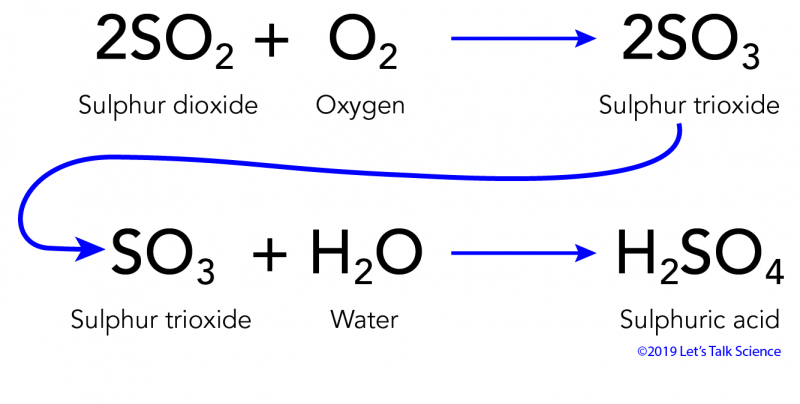
2SO 2 g O 2 g 2H 2 O I 2H 2 SO 4 aq Acid rain occurs when the pH of rain water falls between 20 to 55. Burning coal oil and gas can create the pollutant sulfur dioxide. The nitrogen oxides and sulfur oxides that form acid rain come from both man-made and natural sources.
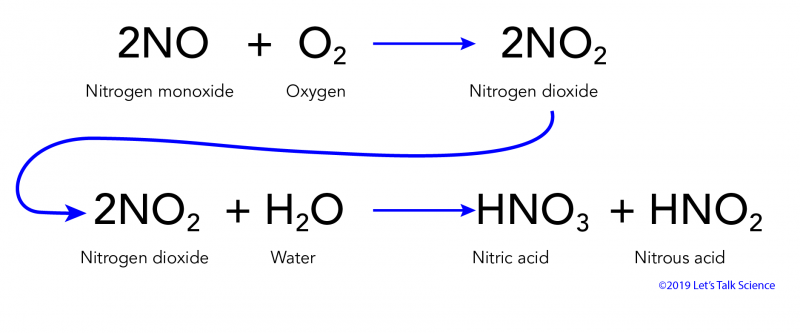
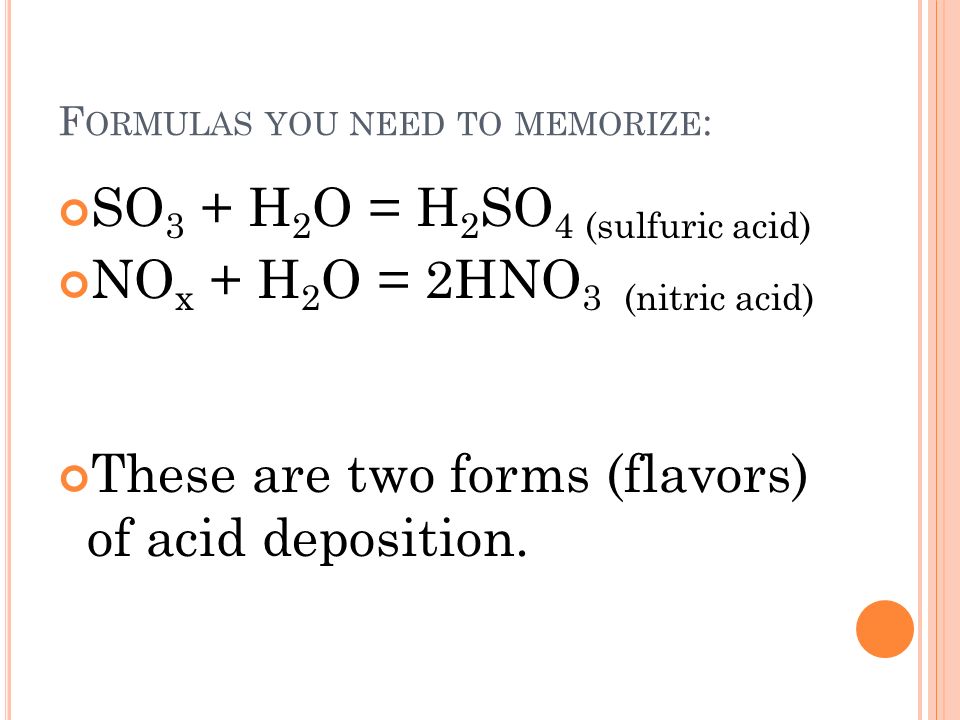
An equation for the formation of nitric acid a component of acid rain is 2NO2 HOH - HNO2 HNO3. The reactions to make either of these are exothermic oxidation reactions. H 2O CO2 H 2CO3.
The ability of H. This is due to the presence of sulphurous acid sulphuric acid and nitric acid in rain water. Sulfuric acid is a strong acid so it readily dissociates in water to give an H ion and an HSO 4-ion Equation 7.
Often soil is slightly basic due to naturally occurring limestone which has a pH of greater than 7. A description of how sulfur impurities lead to the formation of acid rain. Write a balanced chemical equation for this reaction.
This is a simplified representation of this reaction. These substances can rise very high into the atmosphere where they mix and react with water oxygen and other chemicals to form more acidic pollutants known. The chemical equation looks like this.