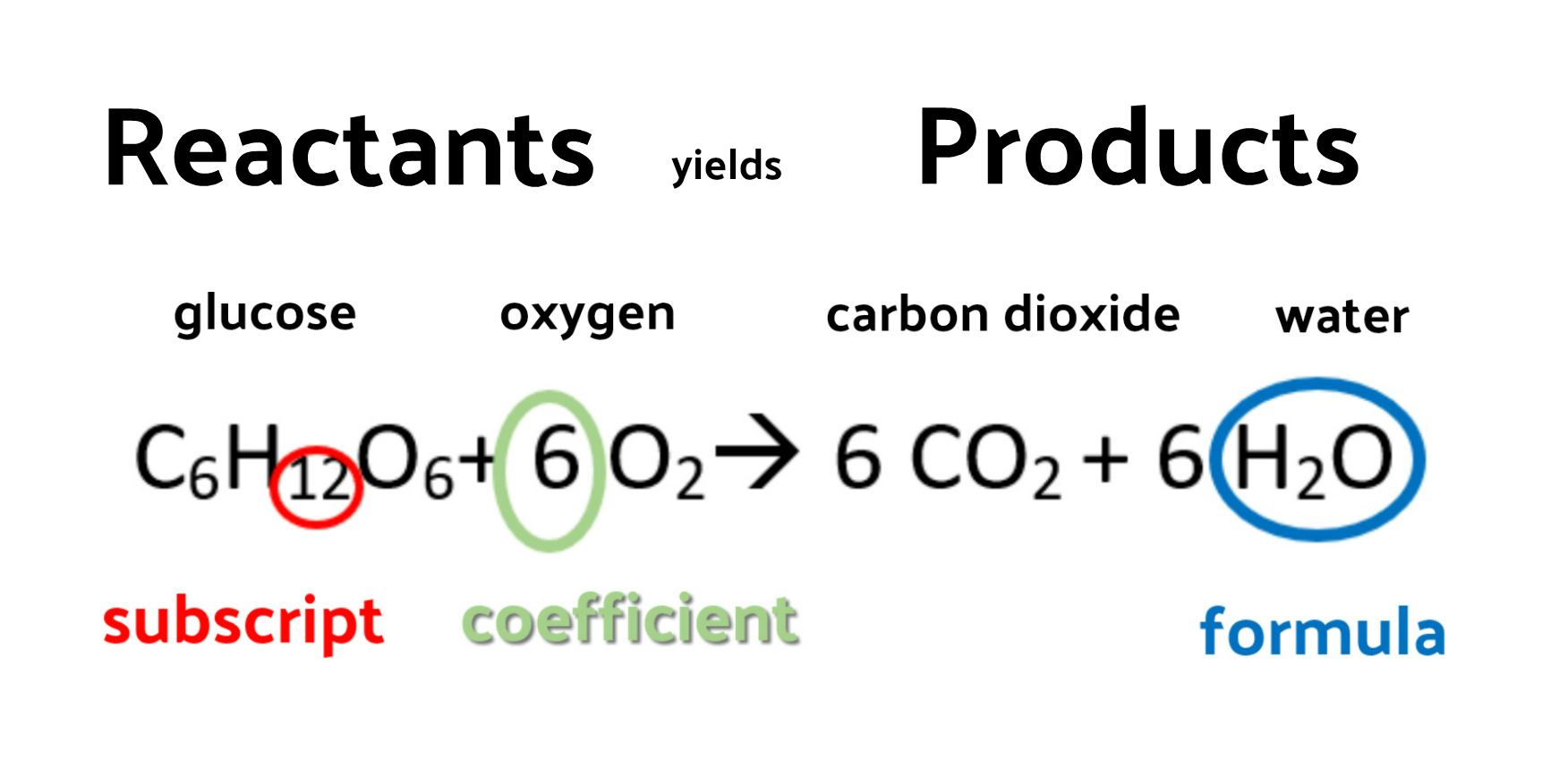
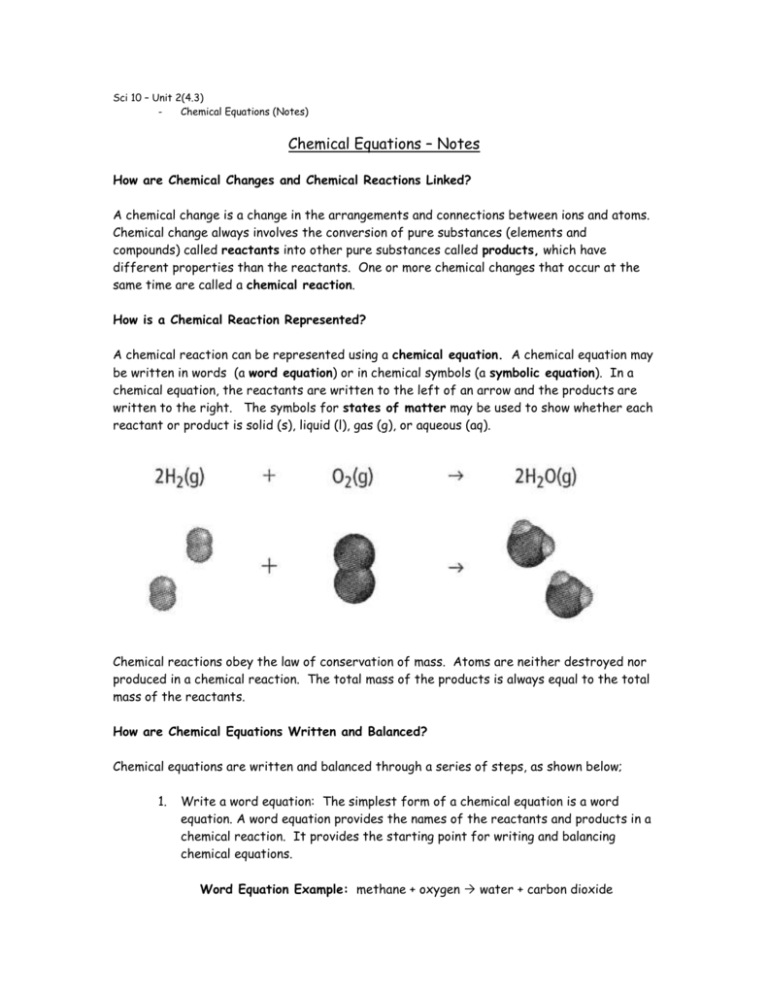
Casual Balanced Equation Of Rusting

Two examples of combustion reactions are.
Balanced equation of rusting. Write the balanced chemical equation for this reactionExample. Asked 031420 Write the balanced equation for the rusting of iron in which iron reacts with oxygen to form ironIII oxide. Rusting of iron refers to the formation of rust a mixture of iron oxides on the surface of iron objects or structures.
Rust is formed when iron reacts with oxygen in moist air. One of the most common iron oxides is iron III oxide known as rust. 4Fe 3O2 g 2Fe2O3.
The formation of rust requires iron water and oxygen. Rust results from a reaction called oxidation in which iron reacts with water and oxygen to form hydrated iron III oxide. This rust is formed from a redox reaction between oxygen and iron in an environment containing water such as air containing high levels of moisture.
The formula is approximately Fe 2 O 3 3 2 H 2 O although the exact amount of water is variable. The transformation of chemical substance into another chemical substance is known as Chemical Reaction. By Staff Writer Last Updated April 16 2020 The oxidation reaction of iron and oxygen to form the substance that is commonly called rust occurs according to this equation.
Rusting is an oxidation reaction. The following chemical equation represents the reaction. Rusting occurs as the corrosion of iron in the alloy steel.
What reacts to form rust. Iron metal reacts with oxygen gas to form rust ironiiioxide. 4 Fe 3 O 2 2 Fe 2 O 3.
:max_bytes(150000):strip_icc()/BalanceEquations2-56a132765f9b58b7d0bcf538.png)