Amazing Define Displacement In Chemistry

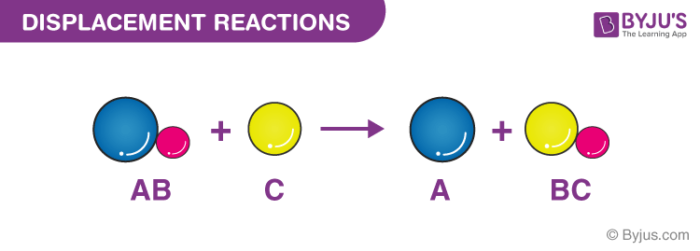
Displacement reactions involve a metal and a compound of a different metal.
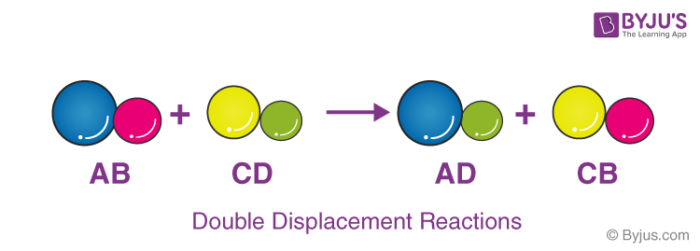
Define displacement in chemistry. Displacement dĭs-plās mənt Chemistry A chemical reaction in which an atom radical or molecule replaces another in a compound. A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products. Those reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions.
Example Fe CuSO4 FeSO4 Cu. In double replacement reactions the positive ions exchange negative ion partners. It is represented as an arrow that points from the starting position to the final position.
Displacement is defined as the act of moving someone or something from one position to another or the measurement of the volume replaced by something else. For example- If an object moves from A position to B then the objects position changes. It can be defined mathematically with the following equation.
The chemical bonds between the reactants may be either covalent or ionic. Displacement reactions Displacement reactions occur when a metal from the electrochemical series is mixed with the ions of a metal lower down in the electrochemical series. It is a vector quantity and has a direction and magnitude.
A substitution reaction is a type of chemical reaction where an atom or functional group of a molecule is replaced by another atom or functional group. A BC B AC. Physics A vector or the magnitude of a vector that points from an.
Many double displacement reactions occur between ionic compounds that are dissolved in water. Refers to the value of the initial position. Single displacement reactions take the form.