Matchless Dilute Sulphuric Acid Reacts With Zinc Granules Formula
Also what happens when zinc metal reacts with dilute h2so4 How will you test the gas evolved.
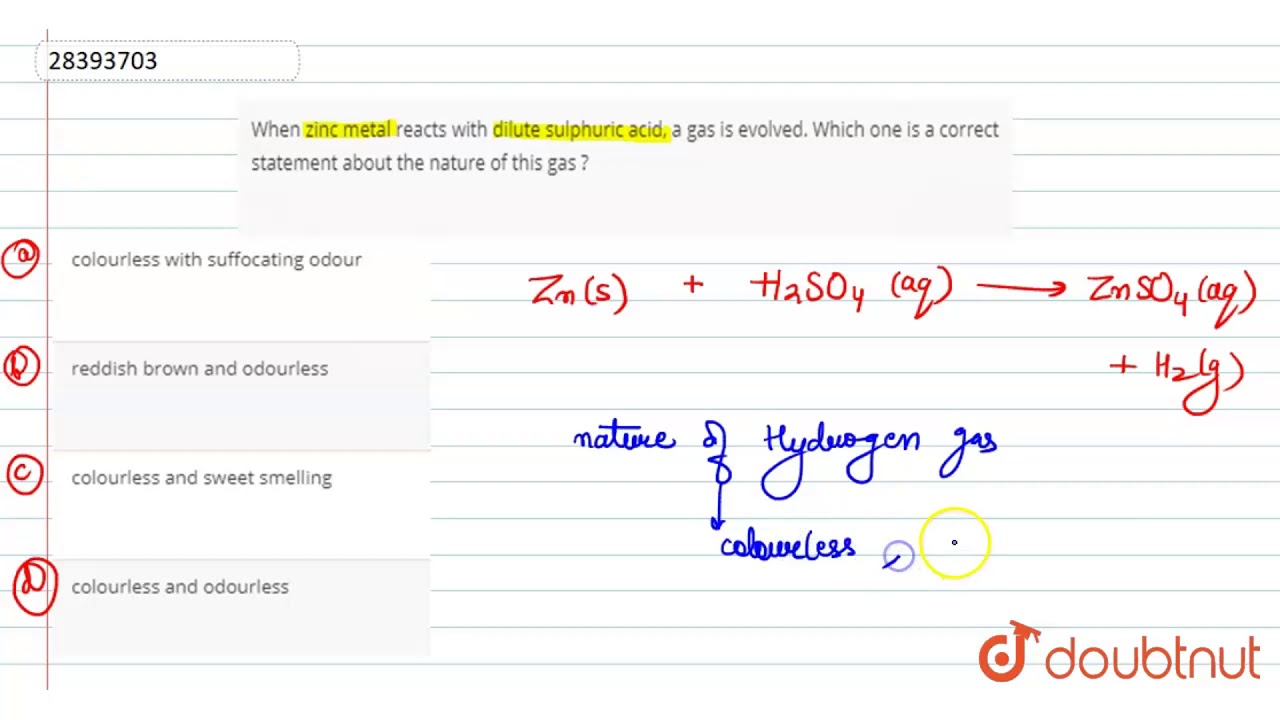
Dilute sulphuric acid reacts with zinc granules formula. A Zinc dilute sulphuric acid Zinc sulphate Hydrogen Zn s H 2 SO 4 aq ZnSO 4 aq H 2 g. Sulphuric acid Zinc Sulphate Hydrogen gas This is an example of displacement reaction of a non-metal by a metal. This can be observed when a match stick that is burnt is bought ear to the test tube.
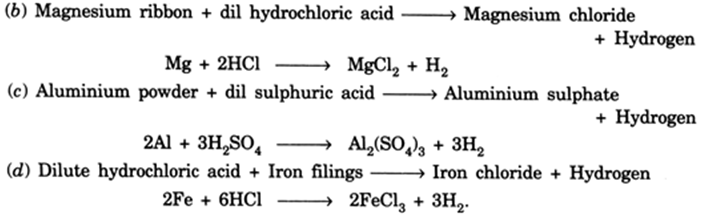
Zn H2SO4 ZnSO4 H2. Click hereto get an answer to your question Write word equations and then balanced equations for the reactions taking place whena dilute sulphuric acid reacts with zinc granulesb dilute hydrochloric acid reacts with magnesium ribbonc dilute sulphuric acid reacts with aluminium powderd dilute hydrochloric acid reacts with iron filings. A Dilute sulphuric acid reacts with aluminium powder.
Acid metal salt Hydrogen gas The Balanced chemical equation is Zn H2SO4 ZnSO4 H2. Hydrogen is a combustible gas and burns with a popping sound. A Dilute sulphuric acid reacts with zinc granules.
When dilute sulphuric acid is poured on zinc granules then zinc being more reactive than hydrogen displaces it from the acid and forms zinc sulphate and hydrogen gas. Test for the gas by placing a lighted wooden splin. Zn 2HCl ZnCl 2 H 2.
When dilute sulphric acid reacts with zinc granules then zinc sulphate and hydrogen gas are formed. In aqueous solution the Zn II ion is present as the complex ion Zn H 2 O 6 2. Zinc Hydrochloric acid Zinc Chloride Hydrogen iii Procedure Some pieces of granulated zinc are placed in the Woulfes bottle and the apparatus is made air tight.
Zinc being more reactive than hydrogen displaces i. That affects reaction rate. How long will the footprints on the moon last.