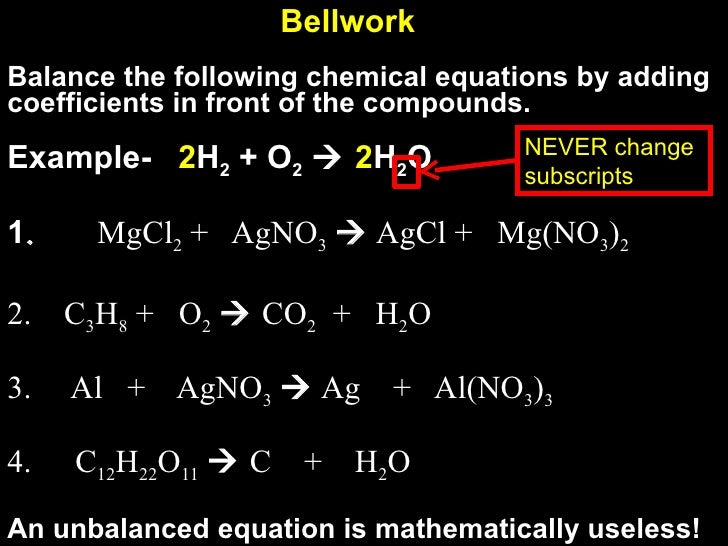
Beautiful Work Examples Of Unbalanced Equation

This means that there are UNEQUAL numbers at least one atom on each side of the arrow.
Examples of unbalanced equation. Therefore this is an unbalanced chemical equation. Practically anything that moves is a result of the exertion of unbalanced forces on it. In the reaction Mg O2 MgO the number of atoms of each element on either side of the arrow is not equal.
The equation is not balanced because in the reactants side there are 2 nitrogen N atoms and 2 hydrogen H atoms. In the products side there are 1 nitrogen N atoms and 3 hydrogen H atoms. An unbalanced chemical equation lists the reactants and products in a chemical reaction but doesnt state the amounts required to satisfy the conservation of mass.
Examples of Balancing Chemical Equations. I knew Id have to eventually clear the 13 2 so I decided to do so right at. The first step to balance the equation is to write down the chemical formula of reactants that are listed on the left side of the chemical equation.
2C 5 H 11 NH 2 O 2--- CO 2 13H 2 O NO 2. Of the NH4OH on the left. By the way a skeleton equation is not wrong it just hasnt been balanced yet.
An equation is balanced by changing coefficients never the subscripts. FeCl3 3NH4OH --- FeOH3 3NH4Cl And if you count up the atoms on each side you will see that this is now. The number of atoms is equal to the product of the coefficient and the subscript.
What is the example of unbalanced chemical equation. For example this equation for the reaction between iron oxide and carbon to form iron and carbon dioxide is unbalanced with respect to mass. How do you balance chemical equations examples.