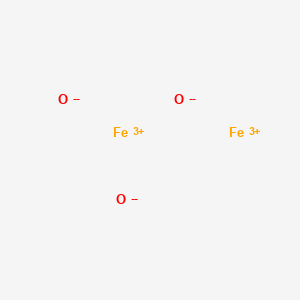Formidable Fe2o3 Lewis Structure
Iron ore is a mineral substance which when heated in the presence of a reductant will yield metallic iron Fe.
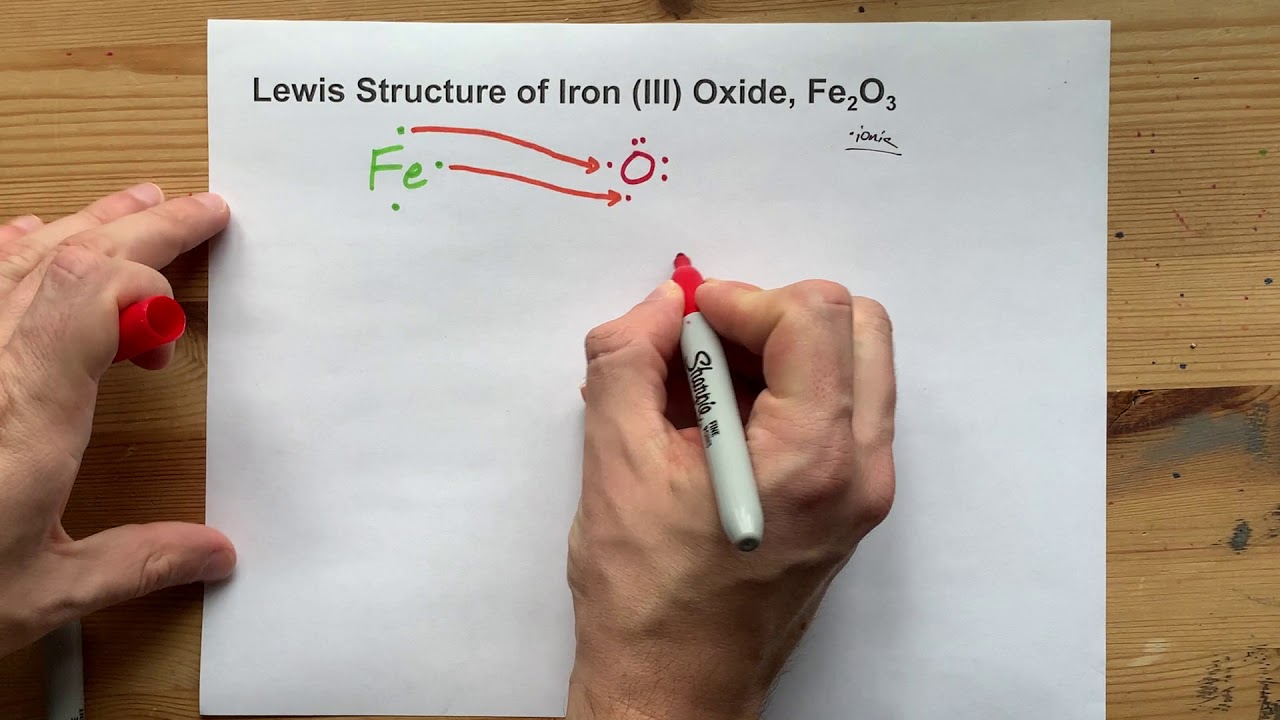
Fe2o3 lewis structure. Draw the correct Lewis structure for Fe2O3. The Lewis Structure of iron III oxide Fe2O3 consists of five ions. The Lewis Structure of iron III oxide Fe2O3 consists of five ions.
Ferric oxide is an iron oxide. It almost always consists of iron oxides the primary forms of which are magnetite Fe3O4 and hematite Fe2O3. The bond formation between oxygen and iron depends on the difference in electronegativity between these two atoms.
Iron III oxide is an ionic compound because it consists of a metal and non-metal. Structure properties spectra suppliers and links for. It is most often seen as rust although this is more properly called hydrated iron.
Draw the correct Lewis structure for Fe 2 O 3. Iron III oxide is an ionic compound because it consists of a metal and non-metal. The Lewis Structure of iron III oxide Fe2O3 consists of five ions.
Iron ore is the source of primary iron for the worlds iron and steel industries. It is one of the three main oxides of iron the other two being iron II oxide FeO the rarer form and iron IIIII oxide Fe3O4 which naturally as. Iron III oxide is an ionic compound because it consists of a metal and non-metal.
Two iron ions with a 3 charge each and three oxygen ions with a -2 charge each. Free unlimited access for 30 days limited time only. Iron II oxides Lewis Structure is among one of the easiest to draw.