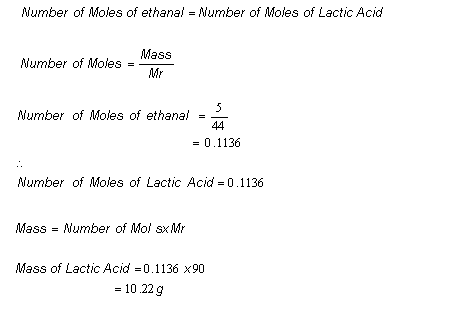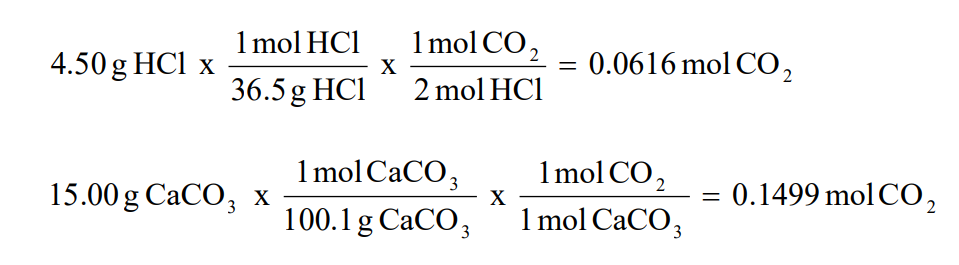Fine Beautiful Percent Yield Of A Reaction Calculator
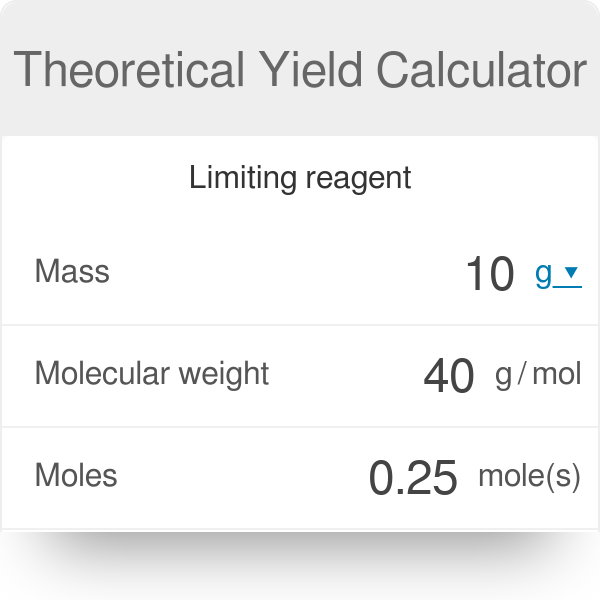
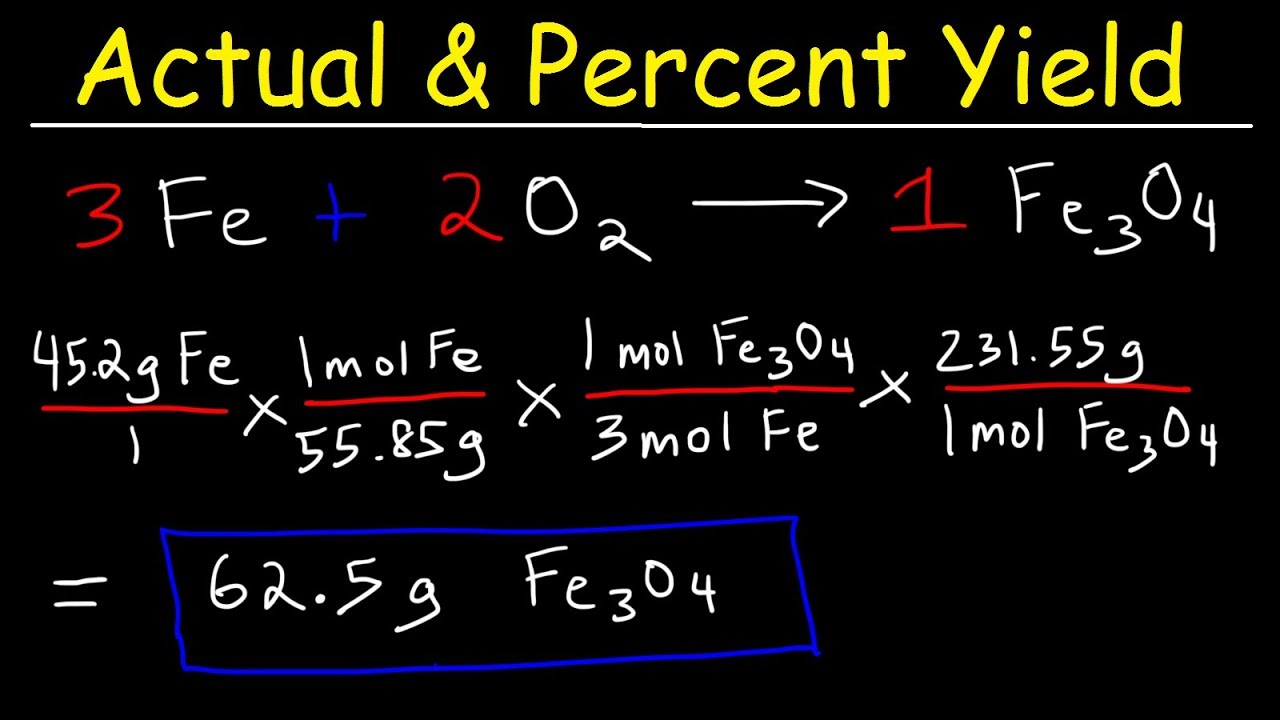
A theoretical yield calculation solves for the maximum amount of product and excess reagent that will be consumed created.
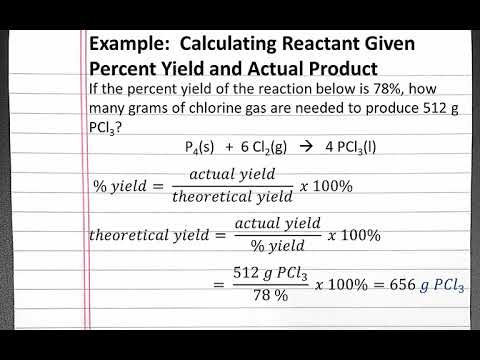
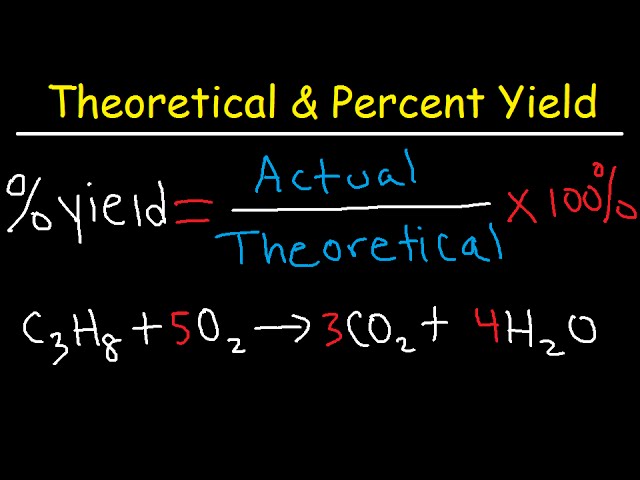
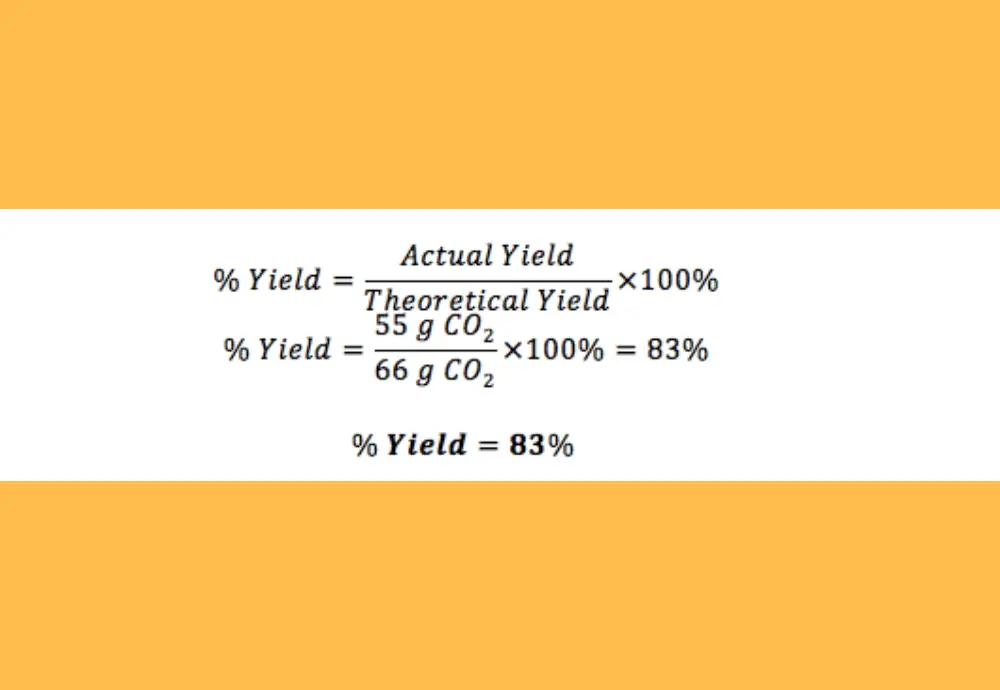
Percent yield of a reaction calculator. Calculate the yield of the following reaction. You can readily perform the percent yield calculation by using this yield percentage calculator or percent yield formula. This is the percent yield.
We use the molar ratio of reactant in a balanced chemical reaction to understand how much product will be created under ideal conditions. In the following reaction 4514 grams of lead reacts with excess oxygen to form 3330 grams of leadII oxide. There are a few steps.
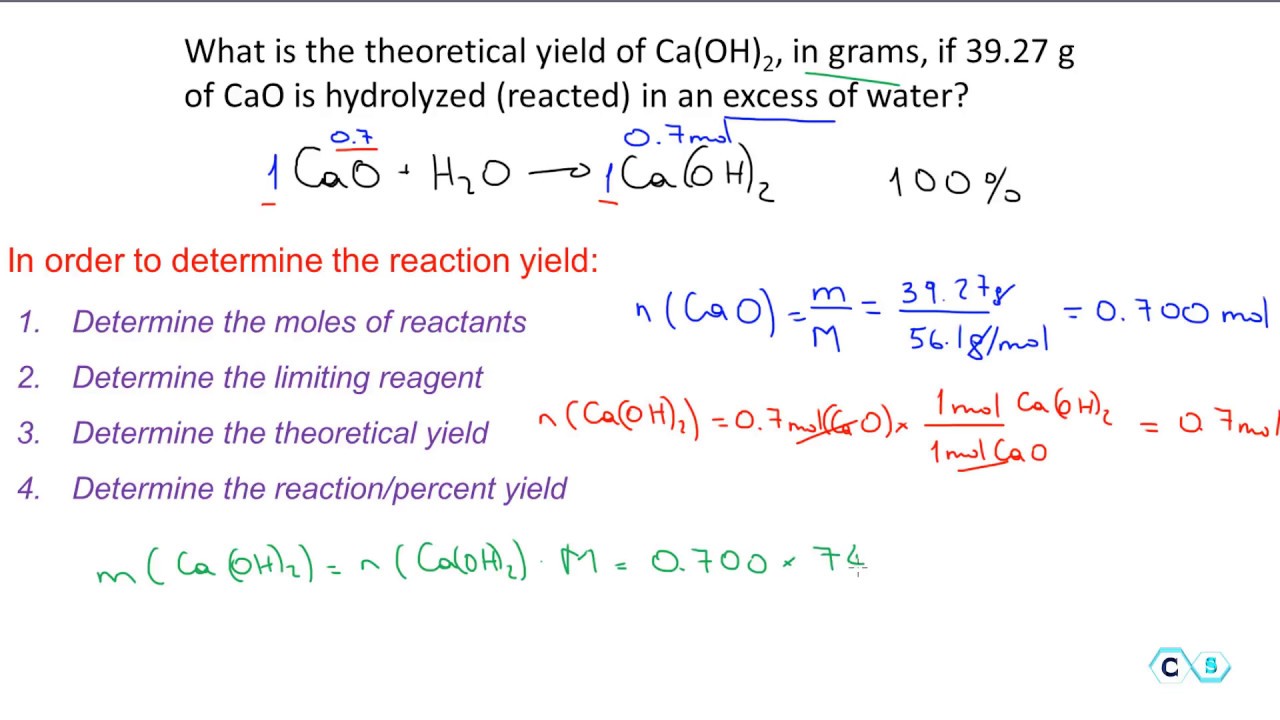
Finally the chemist multiples the unrefined percent yield by 100 to get the final percent he needs to pass along to the soap manufacturer. Now use the following equation. Now calculate the theoretical yield by the help of the above information.
So you find that 8137 is the percent yield. You have isolated 15g of benzil from the reaction of 195 of b enzoin and 10 g of ammonium nitrate A755 B776 C781. Capturing student interest through early coverage of chemical reactions accessible explanations and visualizations and an emphasis on everyday applications the authors explain chemical concepts by starting with the basics using symbols or diagrams and conclude by encouraging students to test.
By following them we can calculate how many grams of product each reagent can produce. If you get a low output check your half initial equation manually or use half life calculator. Percent yield Actual mass of desired product Hypothetical mass 100.
Calculate the percent yield of the reaction. What is the formula to calculate percent yield. Procedure to calculate a reactions.