Supreme Sodium Bromide Balanced Equation

The balanced ionic equation is given below.
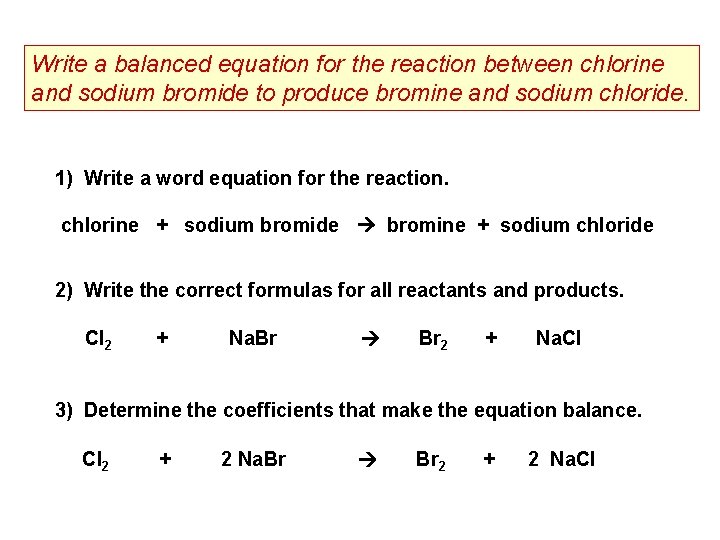
Sodium bromide balanced equation. The balanced chemical equation for the reaction between sodium and water is2 Na s 2 H2O l 2 NaOH aq H2 g We can interpret this to mean. What is a balanced chemical equation for the synthesis of sodium bromide from sodium and bromine. To do soat firstwe have to know the formula of sodium bromidesodium and chlorine.
For a complete explanation watch. Barium chloride sodium sulfate metathesis BaCl 2 Na 2 SO 4 2N aCl BaSO 4. CHEMICAL EQUATIONS II.
Write a balanced chemical equation for this reaction. And the chemical equation. Follow the example set by the sodium bicarbonate.
Write a balanced chemical equation for this reaction. The balanced equation of bromine plus sodium iodide yields sodium bromide plus iodine is2NaI Br2 ------ 2NaBr I2Two moles of sodium iodide react with one mole of bromine to form two moles of sodium bromide and one mole of iodine. What happens when sodium reacts with iodine.
Identify the type of reaction and write a balanced chemical equation for each of the following reactions. The number of sodium bromide molecules involved in the balanced chemical equation. What happens when sodium bromide is mixed with chlorine.
Only change the coefficients these are the numbers in. Hot sodium will also burn in bromine or iodine vapor to produce sodium bromide or sodium iodide. Write the balanced molecular equation for the reaction.