Simple Write A Balanced Equation For The Combustion Of Propane
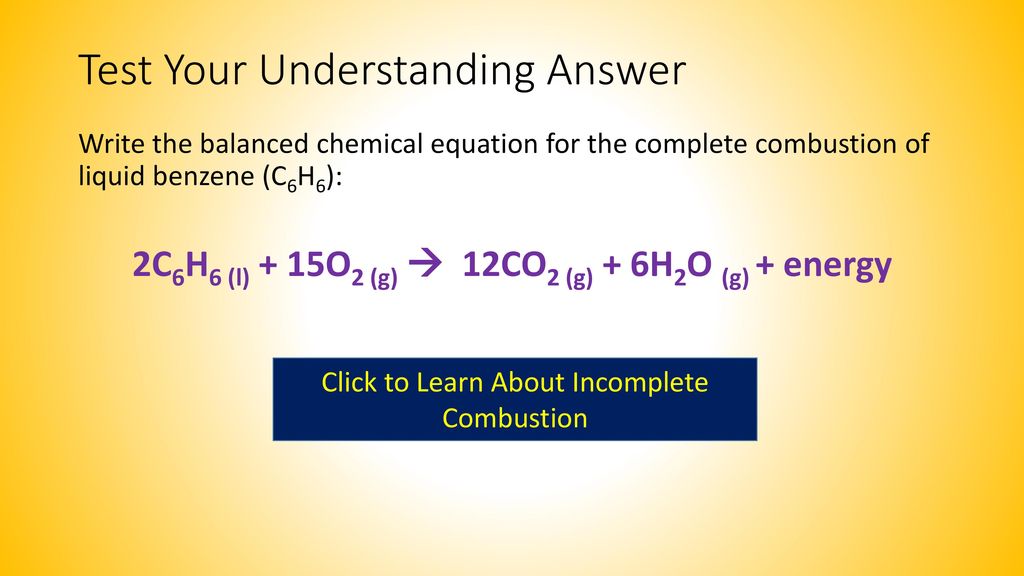
Complete combustion does NOT give carbon monoxid.
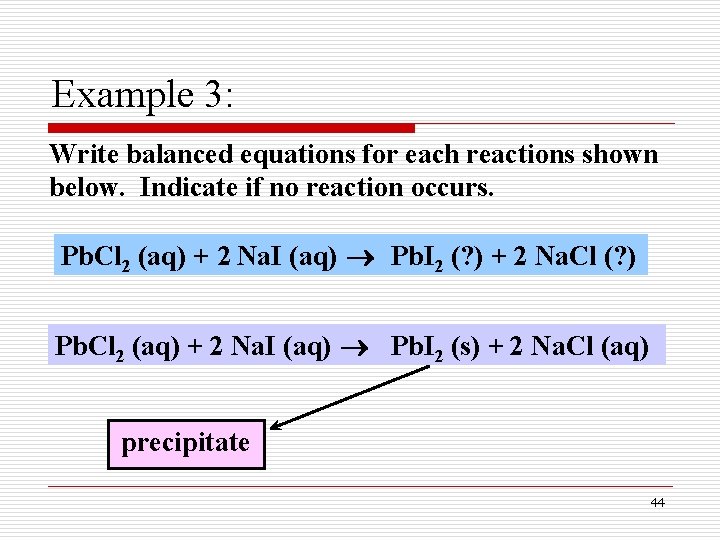
Write a balanced equation for the combustion of propane. Write a balanced equation for the combustion of propane. 2 C3H8 9 O2 4 CO2 2 CO 8 H2O Heat. Both 1-Propanol C3H7OH and 2-propanol react with oxygen O2 to make carbon dioxide CO2 and water H2O.
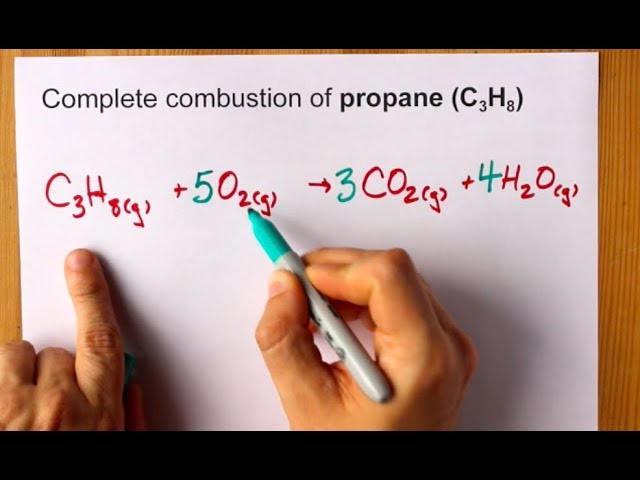
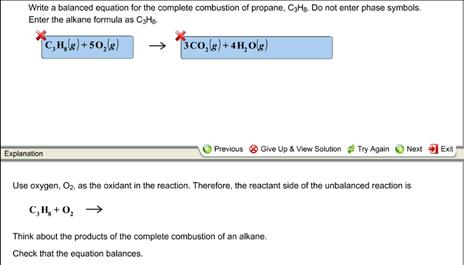
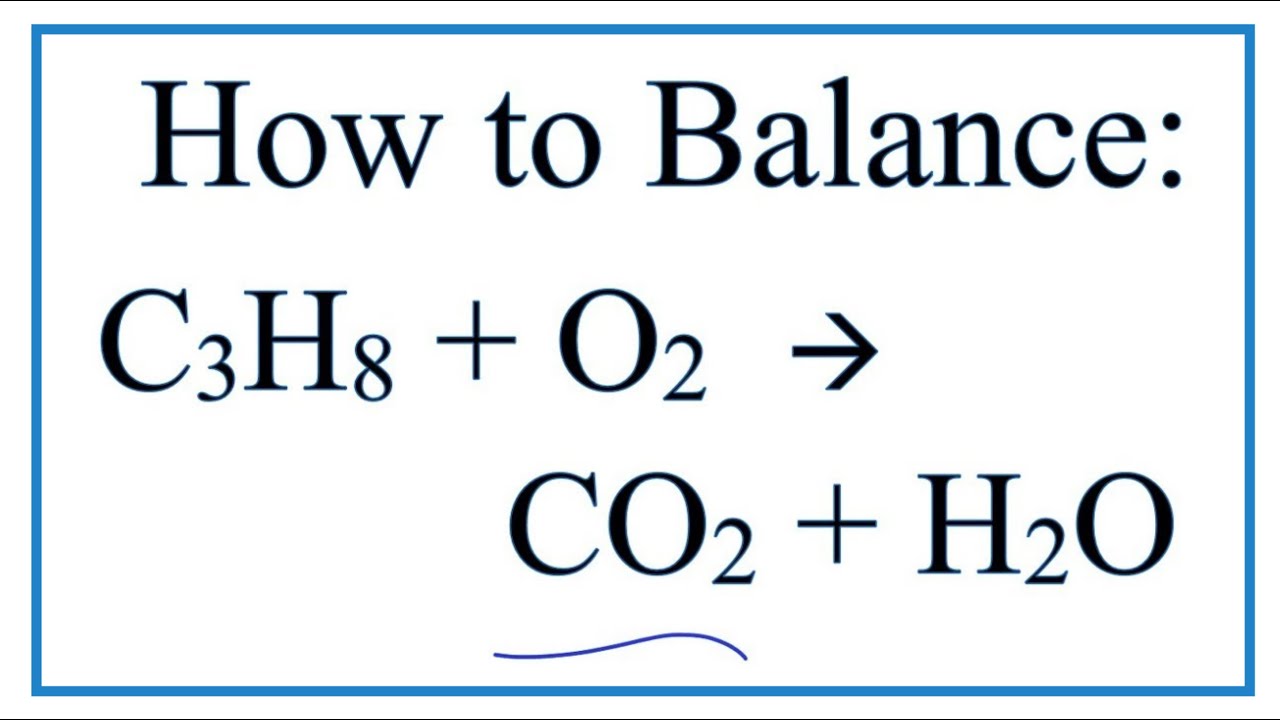
Write a balanced equation for the combustion of gaseous propane C3H8 a minority component of natural gas in which it combines with gaseous oxygen to form gaseous carbon dioxide and gaseous water. Write A Balanced Equation For The Complete Combustion Of Propane CH. C3H85O23CO24H2OThe combustion of pentane C5H12 follows this reaction.
Be sure to include states of matter in your equation - 10084448. Write a balanced chemical equation for the combustion of propane c3h8 if you begin with 100l of propane how many liters of oxygen are required for complete combustion write the balanced chemical reaction for the complete combustion of propane c3h8 you can use shorthand notation a make sure to include all reactants and all products b. The result of incomplete combustion is once again water vapour carbon dioxide and heat.
Propane C 3 H 8 is a hydrocarbon that is commonly used as fuel. The heat generated in the exothermic reaction causes more and more propane to break apart and combine with oxygen in air to produce the end products carbon dioxide and water. Phases Are Optional Equation.
The fuel can be almost anything including methane CH4 propane C3H8 butane C4H10 octane C8H18 or sugar C6H12O6. If not enough oxygen is present for complete combustion incomplete combustion occurs. I answered C3H8 5O2 3CO2 4H2O but it was wrong any idea why.
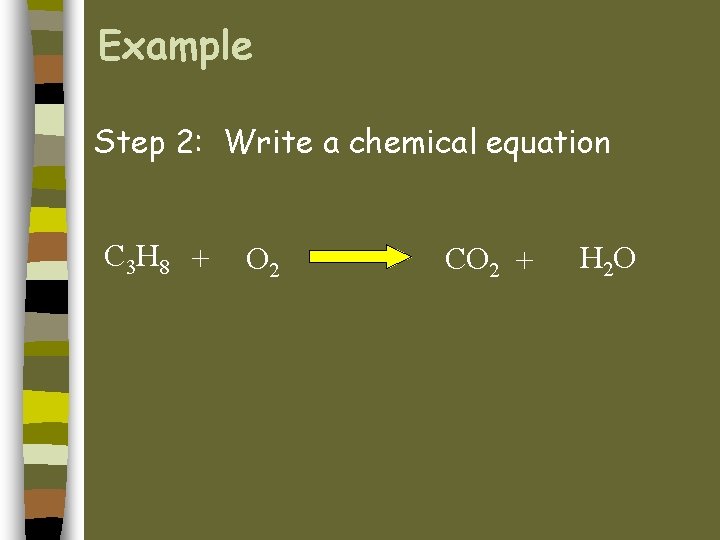
What Is a Combustion Reaction. Write the balanced equation for the combustion of propane. C5H128O25CO26H2OThe combustion of octane C8H18 follows this reaction.