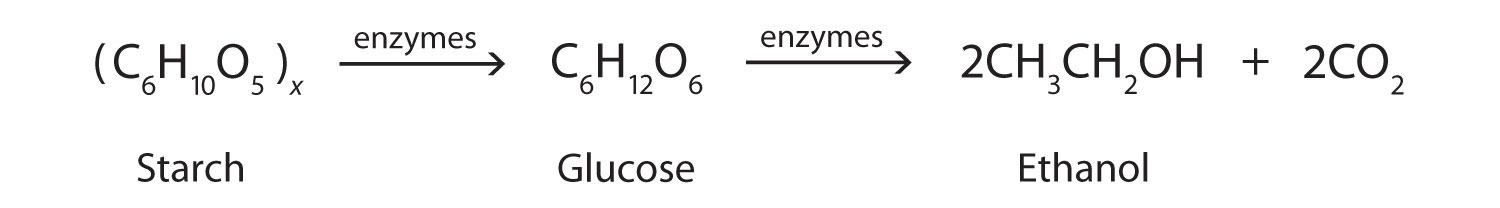Spectacular Balanced Equation For Fermentation

Anaerobic respiration is the breakdown of energy rich molecules without sufficient quantities of oxygen present.
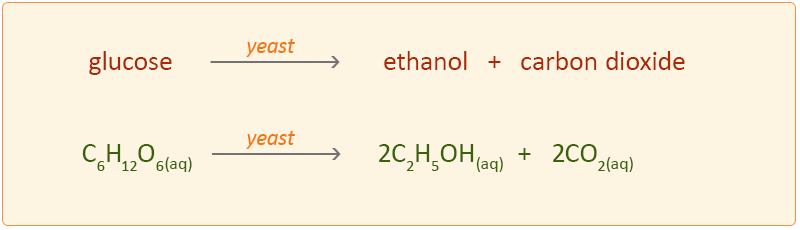
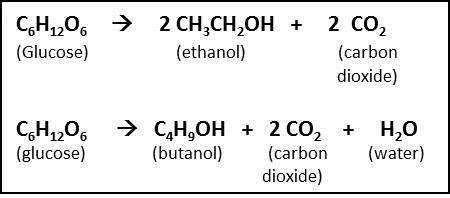
Balanced equation for fermentation. C 6 H 12 O 6 2NAD 2CH 3 -CO-COOH 2NADH 2H. C 6 H 12 O 6 2 C 2 H 5 OH 2 CO 2 Sucrose is a. The main function of fermentation is to convert NADH back into the coenzyme NAD so that it can be used again for glycolysis.
Fermentation occurs in the absence of oxygen anaerobic respiration. 6CO2 6H2O C6H12O6 6O2 light energy is used as a catalyst in this equation Balanced chemical equation for the fermentation of glucose to ethanol. The first step in the fermentation reaction is the conversion of glucose C 6 H 12 O 6 to pyruvic acid CH 3 -CO-COOH using the hydrogen carrier NADH present in the yeast 1.
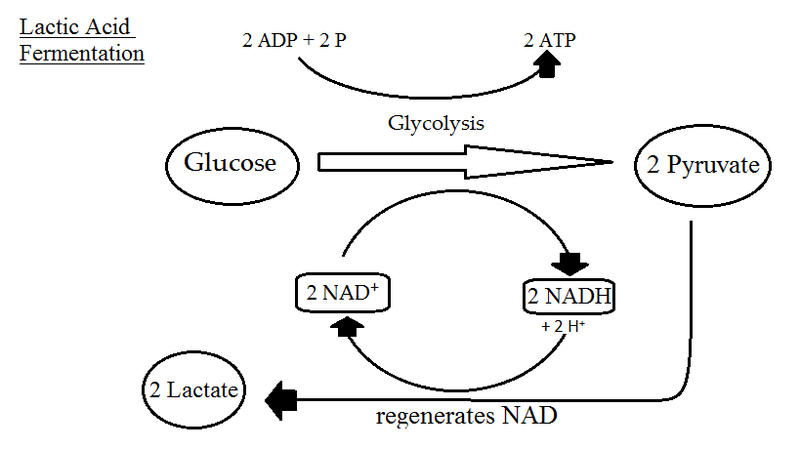
During fermentation an organic electron acceptor such as pyruvate or acetaldehyde reacts with NADH to form NAD generating products such as carbon dioxide and ethanol ethanol fermentation or lactate lactic acid fermentation in the process. What is a well-balanced equation for anaerobic respiration in plants and animals. Chapter 8 Problem 59AYK is solved.
The terminal electron acceptor can use in the yeast cells and muscle cells fermentation aerobic. C6H12O6 ---- 2 C2H5OH 2 CO2 Having determined that 2 moles of ethanol and 2 moles of CO2 are produced for each mole of glucose we need to convert 560 grams or560kg into moles. How cells extract energy from glucose without oxygen.
C6H12O6 aq 2C2H5OH l 2CO2 g yeast acts as a catalyst in this reaction. If youre asking about the balanced equation for complete oxidative metabolism y. Fermentation is generally defined as the conversion of carbohydrates to acids or alcohols.
C6H1206 aq 2 C2H60 aq 2 CO2 g glucose ethanol How many grams of CO2 are formed from 020 mol of glucose. C 6 H 12 O 6 -- 2 C 2 H 5 OH 2 CO 2 glucose -- ethanol carbon dioxide. This requires that you start with a balanced equation for the fermentation reaction.