Impressive Hand Warmer Chemical Equation

There are two types of chemical hand warmers that Im going to look at here.
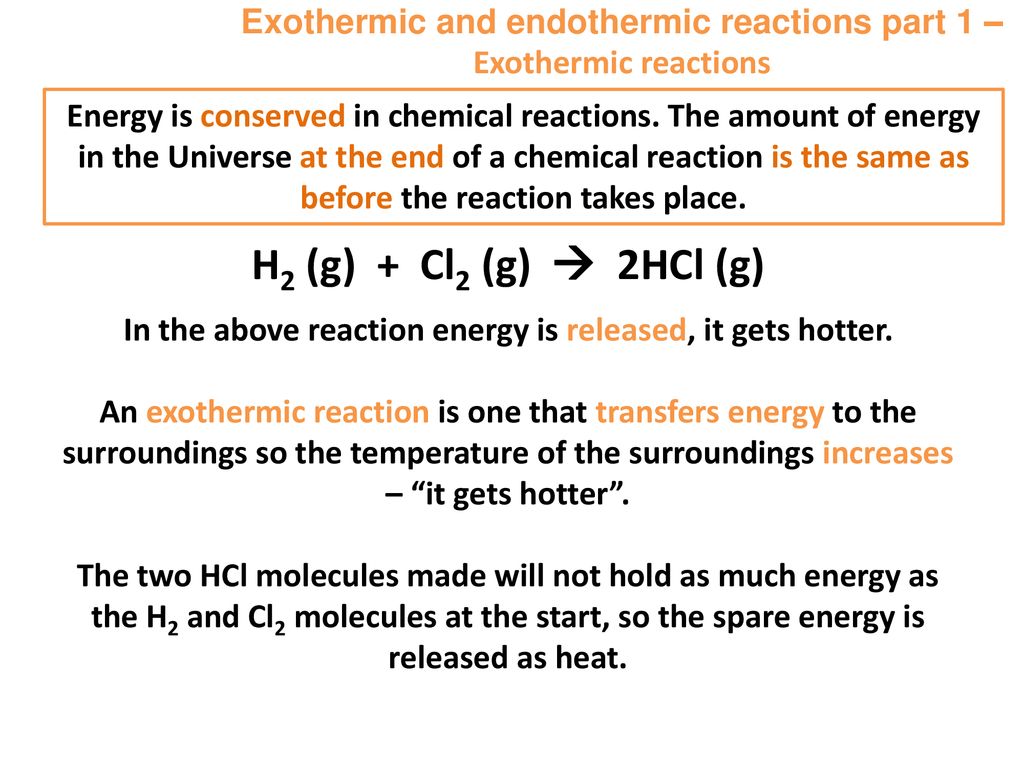
Hand warmer chemical equation. Disposable hand warmers turn up the heat in your mittens by means of an exothermic reaction that in essence just creates rust. These hotties can reach up to 163 degrees Fahrenheit. The first are the more common disposable variety.
7This is how the super saturated hand warmers. How Air Activated Hand Warmers Work. Activated charcoal is a very porous material that holds the water needed for the oxidizing rusting reaction to occur.
Hand warmers you buy usually have a porous pouch containing a mix of iron powder activated charcoal salt and vermiculite. To reset the crystallised pad to its original gel form add each activation lasts up 90 minutes and can em. As the crystals spread the stored heat energy of the solution is released heating the hand warmer up to 54C an exothermic reaction.
Oxygen in the air reacts with this powder to yield iron oxiderustand heat. Popular hand warmers rely on heat-releasing chemical reactions also known as exothermic reactions. The hand warmer can be reset by boiling it in a pan of water to liquefy.
The rusting is a redox reaction and the equation is as follows. Chemical hand warmers produce heat when theyre removed form their airtight plastic wrappers. Each packet contains iron cellulose or sawdust -- to bulk.
These hand warmers utilize the oxidation of iron to form iron oxide. This is a crucial role for the hand warmer if it wasnt for oxygen the rust reaction would not be able to occur and no heat would be produced2 They typically emit on average from 7-10 hours of heat. The iron within the hand warmer mixes with the oxygen in the air and oxides because it gives off heat when it reacts the process is known as an exothermic oxidation.