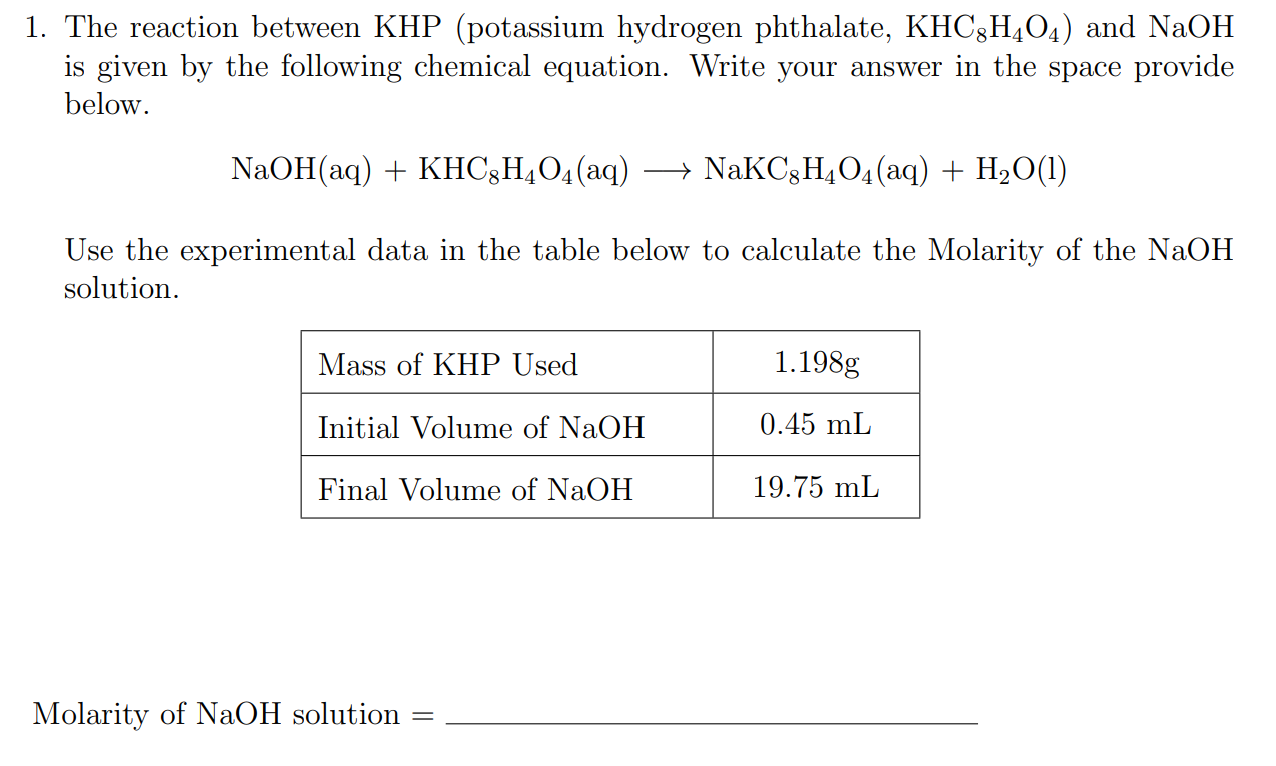
Amazing Chemical Reaction Between Khp And Naoh

What is the chemical equation for KHP and NaOH.
Chemical reaction between khp and naoh. My question is where does the KHP come into this equation. You start with 05100 g of KHP. Click to see full answer.
With a molar mass of 20422gmol. Write the balanced chemical reaction between KHP and NaOH. When KHP and NaOH combine a positive hydrogen ion leaves the KHC8H4O4 and a negative hydrogen atom leaves the NaOH.
Since the reaction between KHP and NaOH is of 11 stoichiometry this means that 0002509 mole of NaOH must have been used. Part 1 The balanced chemical reaction between KHP and NaOH is- KHP NaOH KNaP H2O So 1 mol NaOH neutralises 1 mol of KHP. KHP HKC 8 H 4 O 4.
KHP stands for potassium hydrogen phthalate which has the chemical formula KHC8H4O4. 6 How many moles of NaOH were required to neutralizereact with 0001339 moles of KHP. When these two are allowed to undergo an acid-base reaction they will react.
M NaOH g KHP 1 mol KHP 20423 g 1 mol NaOH 1 mol KHP V L of NaOH 2. From the balanced chemical equation Figure 2 below it should be easy to see that the moles of KHP neutralized equals the moles of NaOH delivered due to the 11 stoichiometry of this acid-base reaction. Hydroscopic is when reagents rapidly absorb moisture from the air.
The titration of NaOH with KHP involves adding NaOH from the burette to a known volume of KHP. In this reaction as well one mole of KHP completely reacts with one mole of NaOH. View the full answer.