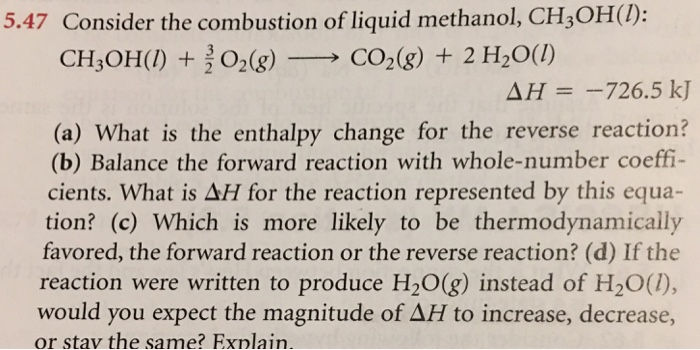Breathtaking Combustion Reaction Of Ch3oh

CH3OH and H2CO were formed as major products from the 100 eV electron-irradiated mixed CH4H2O solid at 10 K.
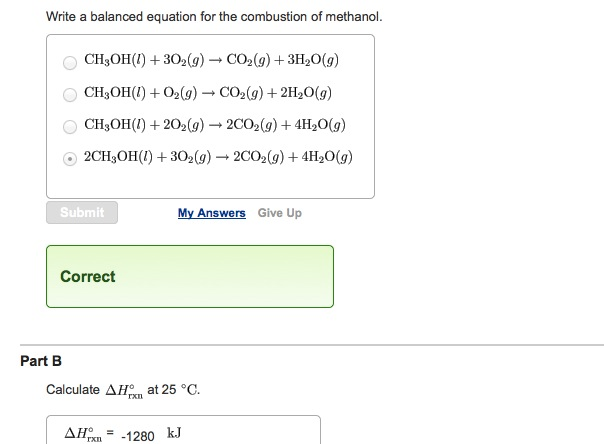
Combustion reaction of ch3oh. Important modifications include recent revisions for the hydrogenoxygen submechanism Li et al Int J Chem Kinet 2004 36 565 an updated submechanism for methanol reactions and kinetic and thermochemical parameter modifications based upon recently published information. The balanced chemical equation for the combustion of liquid methanol in oxygen gas to yield carbon dioxide gas and liquid water is. The most sensitive reaction steps of the methanol mechanism are identified using a global screening method.
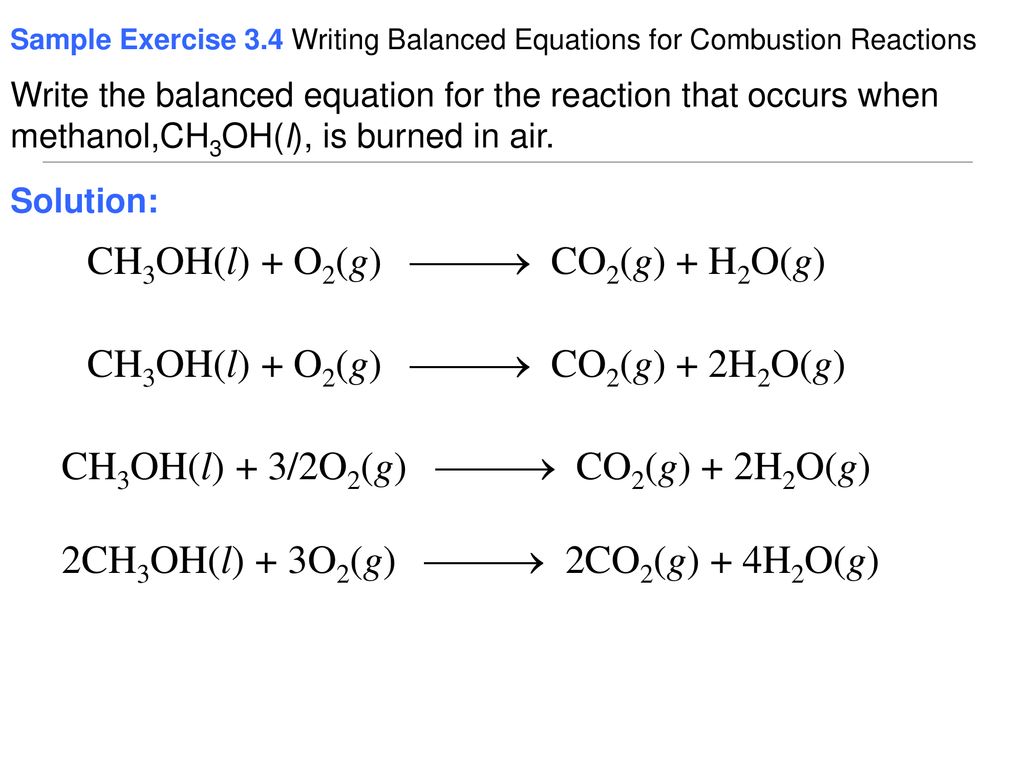
In this video we determine the type of chemical reaction for the equation CH3OH O2 CO2 H2O Combustion of MethanolSince we have a hydrocarbon plus Ox. 2001 for this reaction significantly degrades laminar flame speed predictions for oxygenated fuels as well as for other hydrocarbons. Here we focus on the ignition phase of the combustion and thus we adopt the auto-ignition delay time τ as.
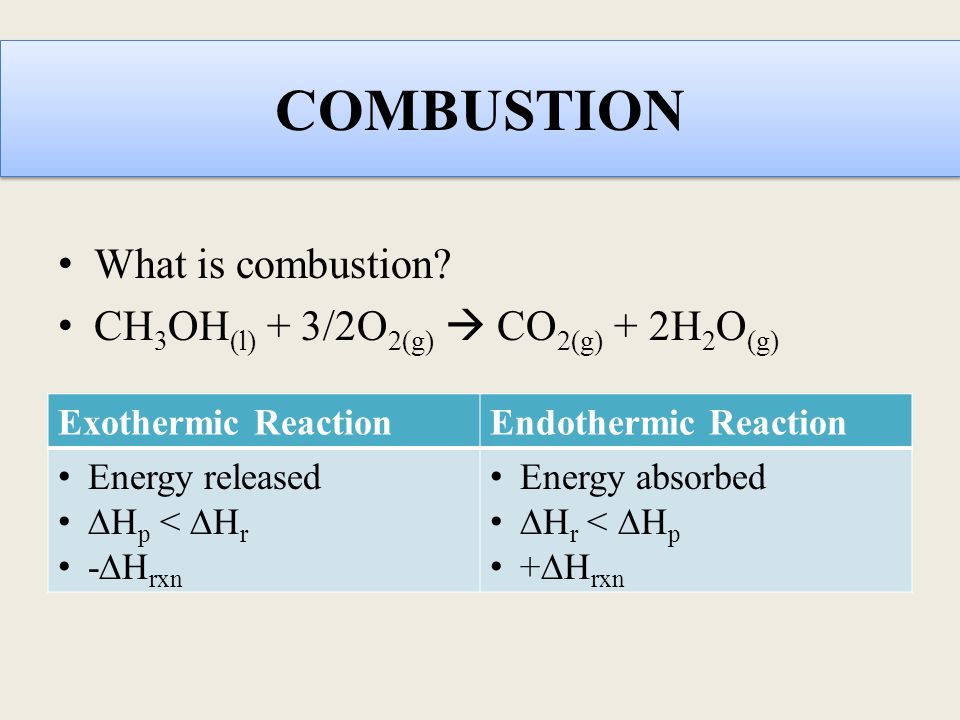
2002 and DeSain et al. The density of liquid methanol is 0792 gmL. Methanol CH3OH burns in oxygen to form carbon dioxide and water.
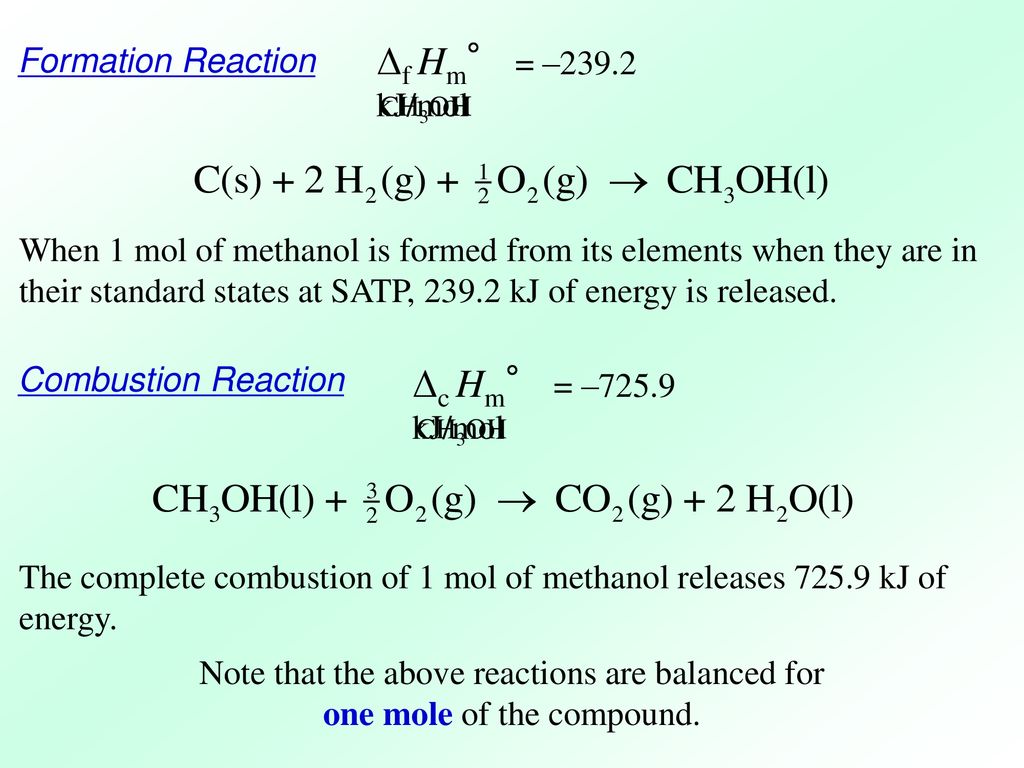
CH3OH and H2CO were formed as major products from the 100 eV electron-irradiated mixed CH4H2O solid at 10 K. Secondly we will total the reactions to obtain the formation reaction of methanol. Top contributors to the provenance of Δ f H of CH3OH l The 20 contributors listed below account only for 783 of the provenance of Δ f H of CH3OH l.
The most complicated formula looks like CH3OH. 2CH3OHl 3O2g 2CO2g 4H2Ol. There found to be two pathways for the formation of methanol with about equal importance ie the recombination reaction.
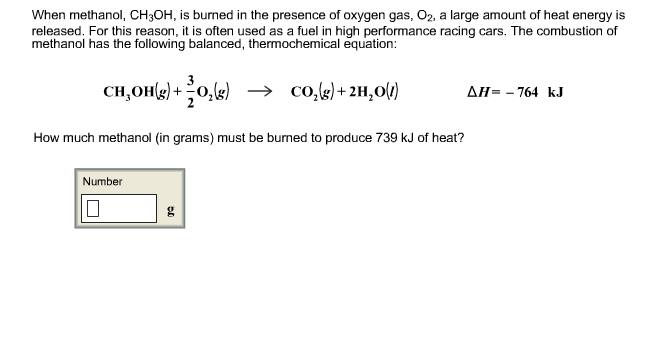
The assumption is the answer is in milliliters mL. Write a balanced equation for the reaction using the AH notation - 2 marks b. Sketch a potential energy diagram include title x and y-axis labels reactants and products endothermic or exothermic - 2 marks Question 4 4 points For the reaction.