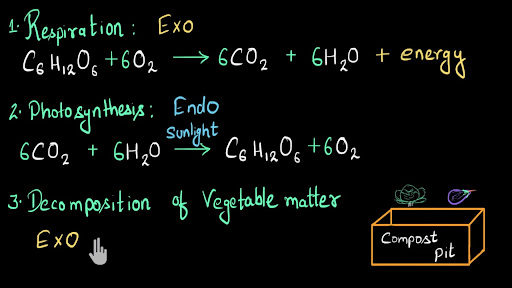Glory Exothermic Formula Examples

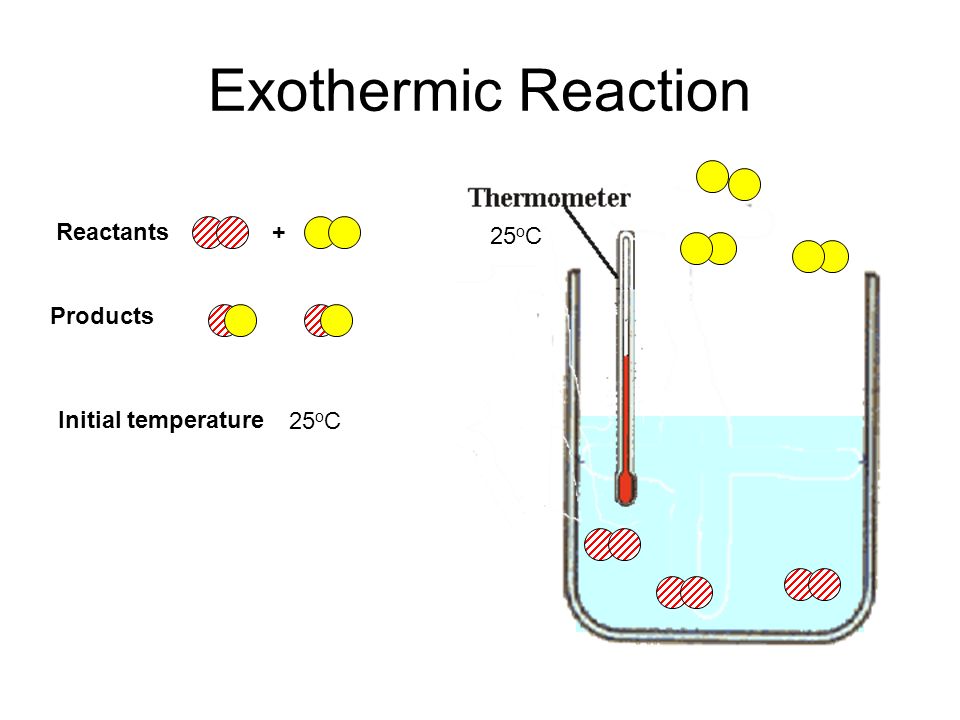
Reactants Products Energy What is an Exothermic Reaction.
Exothermic formula examples. The balanced chemical equations are shown along with the examples 156. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. Combustion refers to a high-temperature exothermic reaction that produces oxidized products.
Reaction between two gases. Examples of exothermic reactions. Mixing sodium Na and chlorine Cl yields sodium chloride NaCl commonly known as table salt.
Combustion the thermite reaction combining strong acids and bases polymerizationsAs an example in everyday life hand warmers make use of the oxidation of iron to achieve an exothermic reaction. A good example of an endothermic reaction is photosynthesis. 4Fe 3O 2 2Fe 2 O 3 ΔH - 1648 kJmol.
Examples of endothermic reactions. 2 Explain why burning wood at a campfire is an exothermic reaction. Heat energy is absorbed from the pan to cook the egg.
An Exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light. 6CO2 6 H2O heat --- C6H12O6 6O2. Exothermic reactions are chemical changes that release heat.
During an exothermic reaction energy is constantly given off often in the form of heat. A particularly important class of exothermic reactions is combustion of a hydrocarbon fuel eg. It explains the flow of heat energy into and out of the system and surroundin.