Casual H2o2 Breakdown Equation

Share Tweet Send Deposit Photos Hydrogen peroxide is an unstable substance.
H2o2 breakdown equation. 2I 2H H2O2 I2 2H2O If the solution is relatively acidic with a pH is less than about 3 the rate of reaction 1 is independent of the pH. The peroxide decomposition rate constants for the additional wastewater treatment plants can be found in. Ordinarily the reaction proceeds too slowly to be perceived but when you pour hydrogen peroxide onto a cut or other surface containing a catalyst it happens much more quickly.
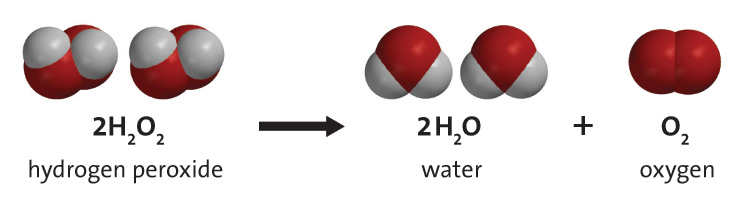
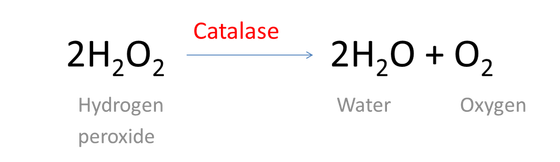
It is used for different reasons including to clean anything that you would clean bleach with. Hydrogen peroxide has a finite shelf-life because over time it naturally decomposes into water and oxygen gas. The breakdown of hydrogen peroxide into water and oxygen is catalysed by catalase enzymes in plants and animals.
Although you are not left with a free radical diatomic oxygen is still an extremely powerful oxidizing agent which is the same reason rust forms on metals. The rate constant for the case of D constant is 00023 min-1 with D 032 mgL and an MSE 00135. In the presence of light the UV light from the sun catalyzes the reaction H X 2 O X 2 spontaneously decomposes into water and oxygen.
H272 H302 H332 H302 H315 H318 H317 P280 P302 P352 P305 P351 P338 H302 H318. The calculated H2O2 decomposition rate constant D xC0 is 000160 min-1 with x 015 indicating that 15 of the dosed hydrogen peroxide is taken up by oxidant demand. For example hydrogen peroxide decomposes to form water H2O and oxygen gas O2.
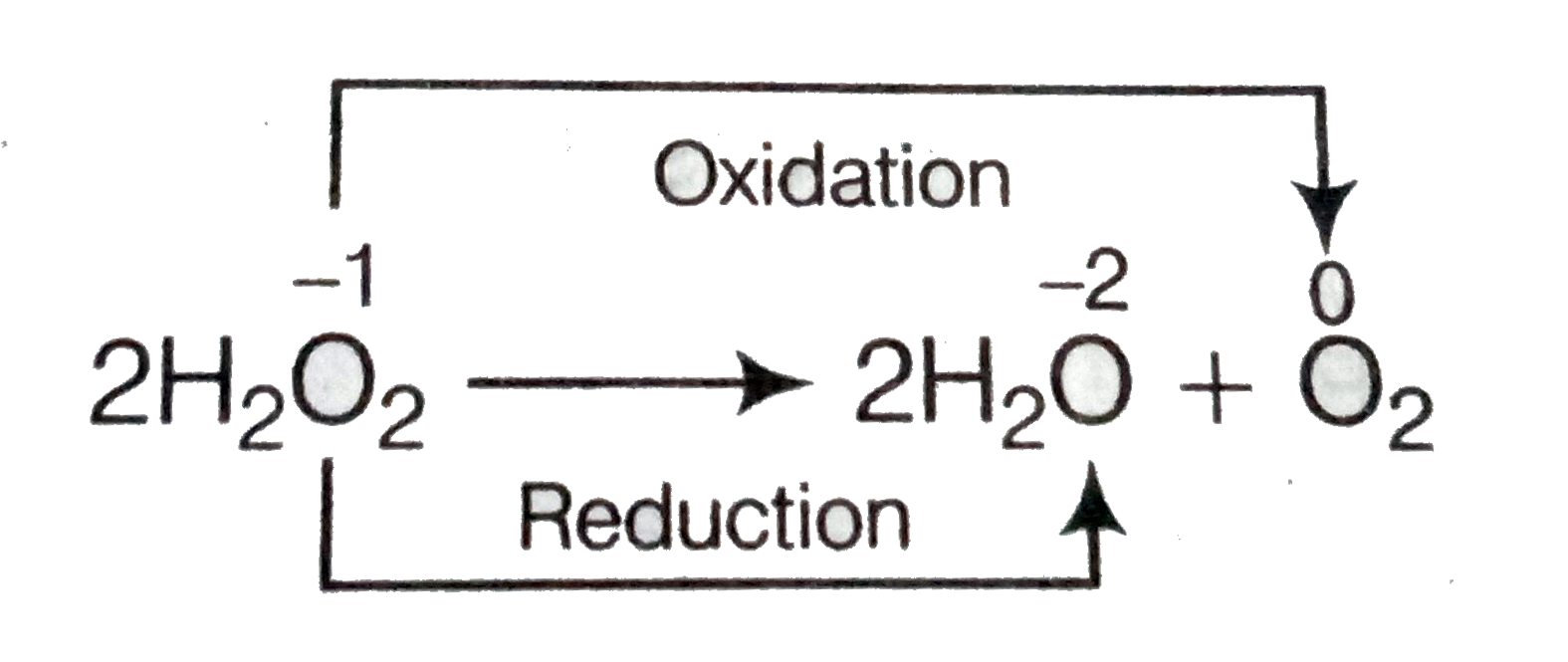
Although this will take a while UV rays from sunlight as well as warm conditions can actually catalyse the decomposition reaction. This is an experiment most students in chemistry lab are familiar with. This is in fact a disproportionation reaction because the OI in peroxide has been reduced to water O I.
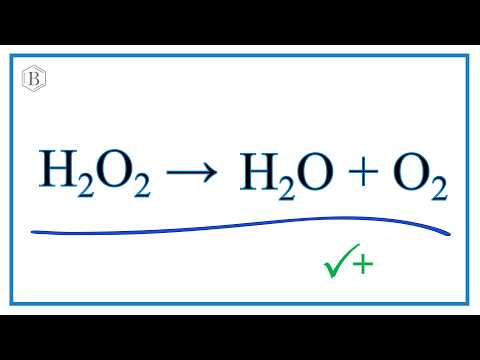
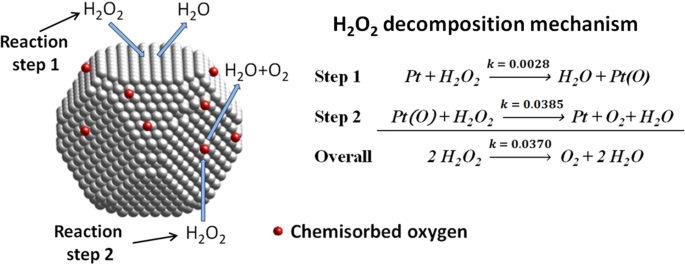
Hydrogen peroxide occurs in important metabolic reactions such as during the action of oxygenases in glyoxysomes and peroxisomes during photosynthesis in choloplasts and during the synthesis of lignin in the apoplast Asada 1992. We need a chemical equation. The balanced equation of the decomposition reaction of hydrogen peroxide is that 2H2O2 decomposes into the products 2H2O O2 g.