Recommendation Lithium Reaction With Cold Water

Solubility in water is related to the ionic nature and size.
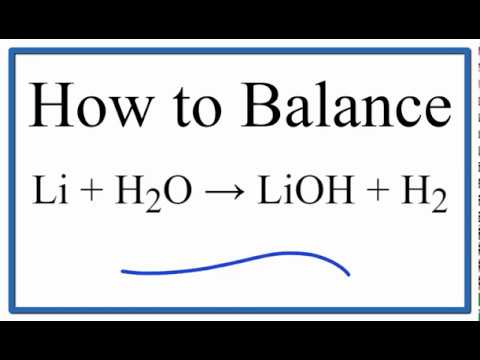
Lithium reaction with cold water. Lithium reacts intensely with water forming lithium hydroxide and highly flammable hydrogen. Reactions of alkali metals with water All the alkali metals react vigorously with cold water. The colourless solution is highly alkalic.
Smaller ions have greater charge density and can be solvated by more water molecules. Write the equation for the formation of lithium. Lithiums density is only about half that of water so it floats on the surface gently fizzing and giving off hydrogen.
The alkali metals such as sodium potassium and lithium react with water to produce heat and flammable hydrogen gas which can ignite or combine explosively with atmospheric oxygen. 26 Votes GROUP 1. 45 618 Views.
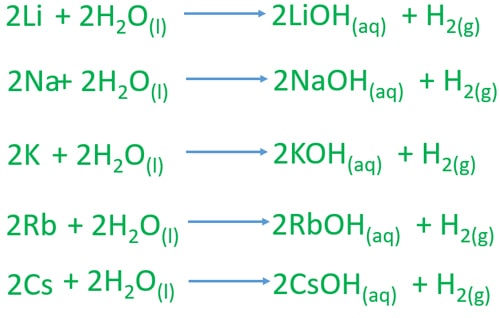
2 Lis 2 H2O - 2 LiOH aq H2g. It gradually reacts and disappears forming a colourless solution of lithium hydroxide. Part of NCSSM CORE collection.
Write the reaction of magnesium with steam. Hazel and Emilia demonstrate the reaction of group 1 metal lithium with water. This video shows the physical properties of Li metal and its reaction with water.
Water-sensitive chemicals are those that react violently with water. They get softer and more reactive as you move down the period from lithium to sodiumto potassium. The solution produced by the.