Divine Simple Chemistry Equations
This side represents the elements which are used for.
Simple chemistry equations. You may think of huge explosions or smoking test tubes when you hear the term chemical reaction. You have to get this right because chemistry will be a nightmare to you if you find equations a mystery - it is an essential tool in understanding chemistry. It shows the number of units of each substance involved.
C F - 32 x 5 9 Converting C to F. A chemical equation is a short-hand way to represent the components of a chemical reaction. The chemical equation has the products on the right side while the reactants are written on the left side.
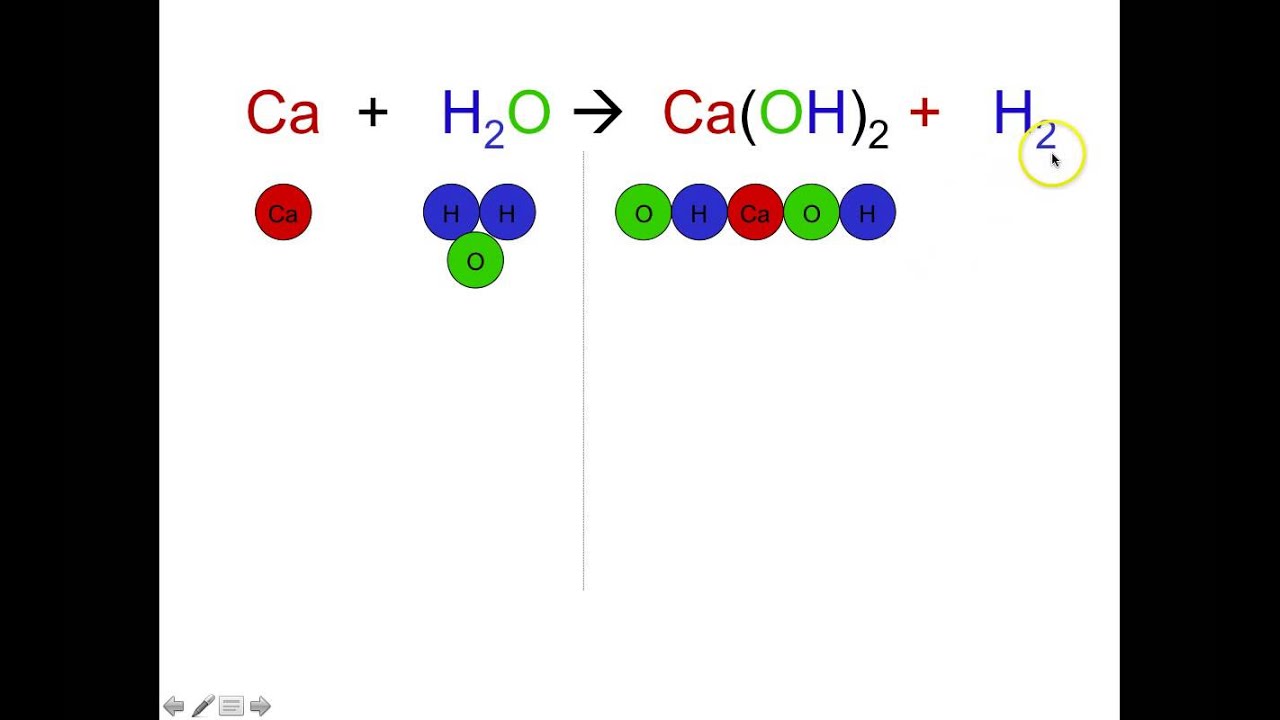
An easy method for beginners to learn how to balance chemical reaction equations using simple drawings. As the name implies simple reactants make or synthesize a more complex product. This means you must always begin and end with the same number of.
How to write symbol equations for simple chemical reactions. A chemical equation is a symbolic representation of a chemical reaction in which the reactants and products are denoted by their respective chemical formulae. D m v Converting F to C.
K C 27315 Percent composition of an element n x molar mass of element molar mass of compound x 100. Easy Equation Balancing Examples. On the left side of the arrow you will find the reactant side.
Balancing Simple Chemical Equations The Law of Conservation of Mass says that atoms are neither created nor destroyed in a chemical reaction. Both of them are separated by an arrow. One of the main types of chemical reactions is a synthesis or direct combination reaction.













