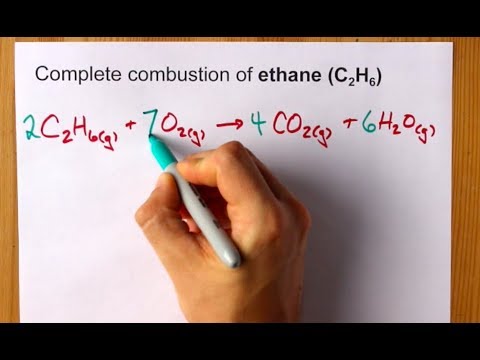
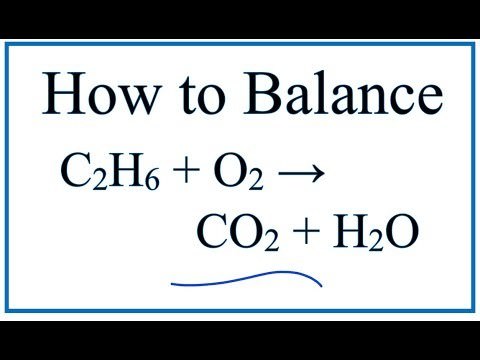
Matchless Balanced Combustion Reaction Of Ethane
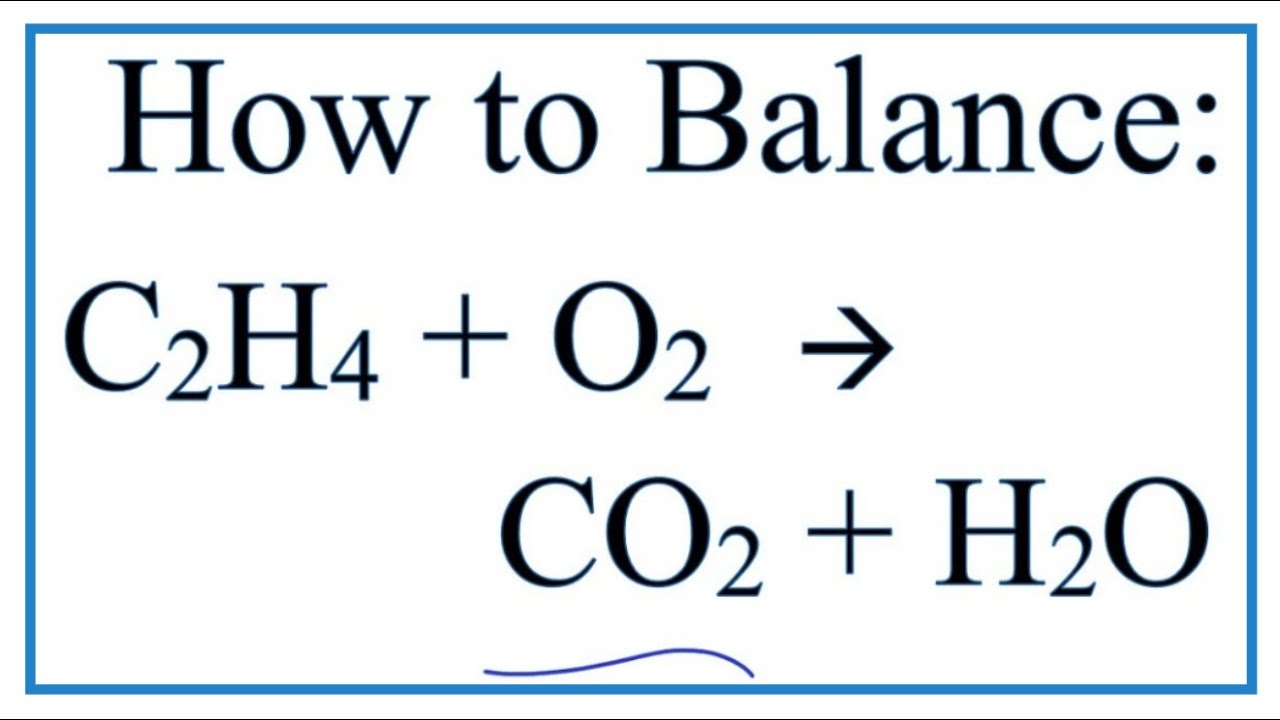
There are 2 carbon atoms from ethene and only one carbon atom from carbon dioxide CO2 C O 2.
Balanced combustion reaction of ethane. A Treatment with sodium hydroxide b Treatment with aqueous K O H c Treatment with N a O H C a O d Halogenation e Reaction with acidified K 2 C r 2 O 7. Complete combustion does NOT give carbon monoxide or sootCheck me out. How to Balance an Ethane Combustion Reaction.
We therefore introduce a. A total of 218 contributors would be needed to account for 90 of the provenance. The reaction also has a negative enthalpy change ΔH value.
Which type of flame is produced when ethane burns. Ethane C2H6 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O. Methane burns readily in air.
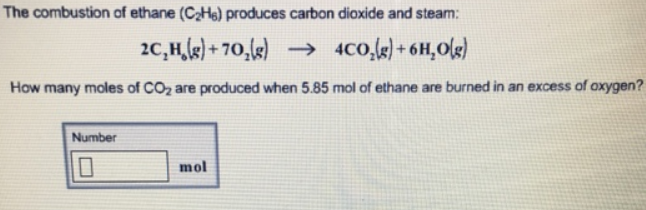
The general reaction will be. The balanced reaction for the combustion of ethane is shown in the table below You allow 3 2 mol of ethane C_2H_6 to react with 4 4 mol oxygen O_2 Complete the following ICF table to indicate the amounts of all chemical species for the initial change and final conditions Drag the appropriate amounts to their respective targets. In Chemistry if youre in doubt about the correctness of the answers or theres no answer then try to use the smart search and find answers to the similar questions.
Fe Au Co Br C O N F. Arrange the reactions given below in proper sequence for the conversion of ethane to methane. During the combustion of 500 g of ethane C2H6 355 kcal is released a Write a balanced.
What is the balanced equation for the combustion of ethane. The balanced chemical equation underneath each reaction description and answer the questions. Use uppercase for the first character in the element and lowercase for the second character.