Favorite What Causes The Formation Of Rust In The Iron Nails
The rusting of iron is characterized by the formation of a layer of a red flaky substance that easily crumbles into a powder.
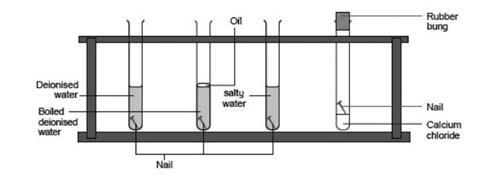
What causes the formation of rust in the iron nails. The nails rust faster in water than in dry air because the liquid allows ions charged particles like Fe and OH- to form and migrate around. Then the water will begin to break down into hydrogen and oxygen. The chemical component of rust is iron oxide.
Rust forms when iron or its alloys are exposed to moist air. If you keep the iron in the acid a relatively short time it will activate the iron and it will rust very rapidly oxidizing the iron to ferric oxide. Water can combine with carbon dioxide in the air to form carbonic acid a weak acid.
Rust is the common name of the chemical called iron oxide. The causes of corrosion require the presence of water and oxygen. The thinner the metal the better the chance that rusting will occur.
Rusting is an oxidation reaction. It turns out what we call rust is a chemical process that combines iron fe and oxygen o to form iron oxide. Here is the word equation for the reaction.
You can get ferrous oxide in some cases. Rustis a general term for iron oxides formed by the reaction of iron with oxygen. Free oxygen reacts with dissolved iron to form iron oxide and iron oxide is rust.
When this acidic solution reaches iron two reactions occur. Technically its iron oxide hydrate because pure iron oxide isnt rust. There is enough oxygen in.