Simple Exothermic Reaction Calculation

Calculate Rate Of Reaction For Highly Exothermic Semi Batch Reaction - posted in Industrial Professionals.
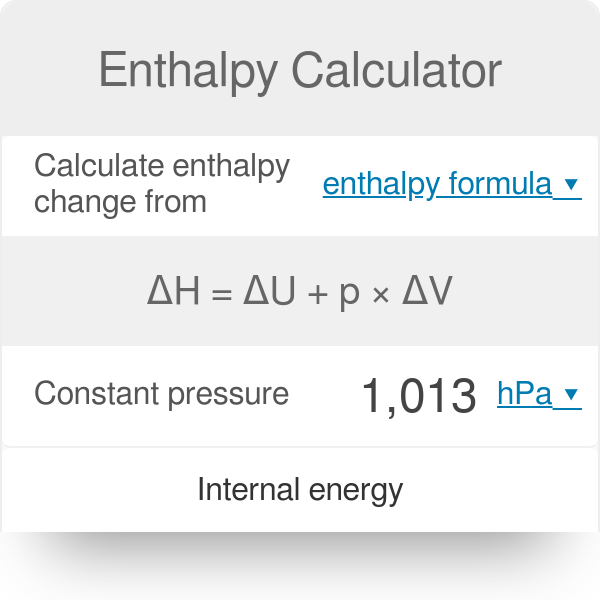
Exothermic reaction calculation. How to calculate the energy transfer change for an exothermic reaction. The purpose of this calculator is to calculate the value of the enthalphy of a reaction delta H or the Gibbs free energy of a reaction delta G. If q is positive the reaction is endothermic ie absorbs heat from its surroundings and if it is negative the reaction is exothermic ie releases heat into its surroundings.
For an adiabatic exothermic reaction the temperature profile. The minimum cold utility remains the same because no heat transfer across Pinch occurs. Energy Calculations where the energy values appear in an equation An exothermic reaction releases energy.
An exothermic reaction is a heat source. By using this website you agree to our Cookie Policy. I have to calculate the order of reaction and reaction kinetics for a reaction which needs to be run in semi batch mode due to high exothermicity.
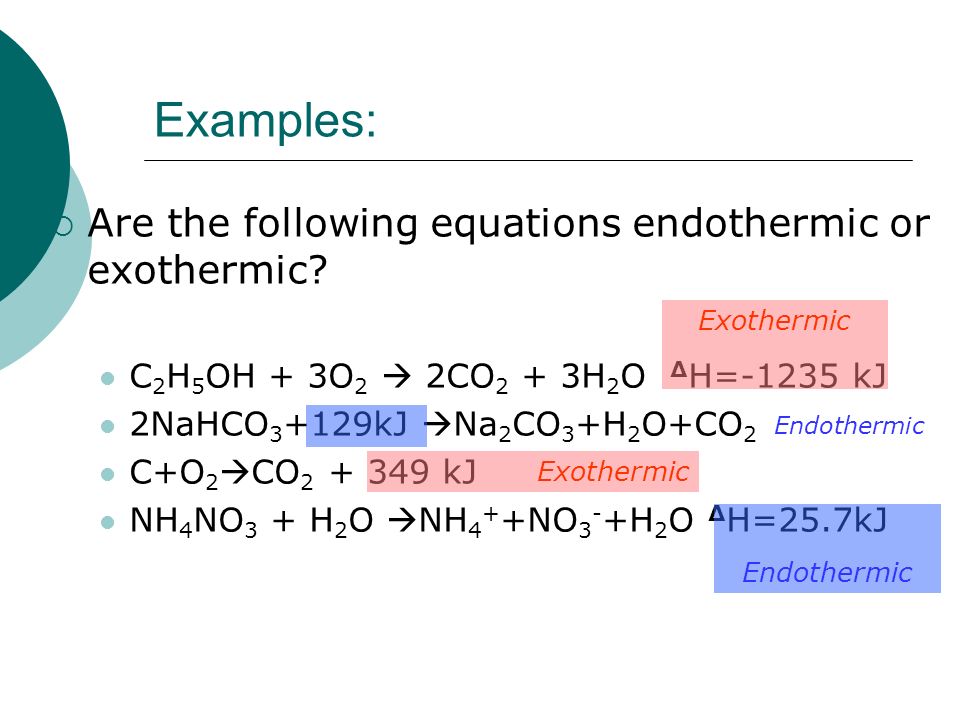
Any increase in temperature indicates an exothermic reaction. In an exothermic reaction change in enthalpy ΔH will be negative. Note these are negative because combustion is an exothermic reaction.
If the enthalpy change listed for a reaction is negative then that reaction releases heat as it proceeds the reaction is exothermic exo- out. Exothermic Reactions Q-value of DT fusion reaction. The equations above are really related to the physics of heat flow and energy.
Nitrogen triiodide is unstable and reacts exothermically when agitated. Enthalpy has units of kJmol or Jmol or in general energymass. When methane gas is combusted heat is released making the reaction exothermic.